Acetone cyanohydrin
- CAS NO.:75-86-5
- Empirical Formula: C4H7NO
- Molecular Weight: 85.1
- MDL number: MFCD00004455
- EINECS: 200-909-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
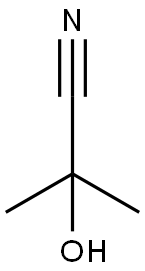
What is Acetone cyanohydrin ?
Description
Acetone cyanohydrin is an organic compound used in the production of methyl methacrylate, the monomer of the transparent plastic polymethyl methacrylate (PMMA), also known as acrylic. Acetone cyanohydrin is a flammable colourless liquid with a faint odour of bitter almond. It is incompatible with sulphuric acid. Acetone cyanohydrin readily undergoes decomposition by water to form hydrogen cyanide and acetone. Cassava tubers contain linamarin, a glucoside of acetohydrin, and the enzyme linamarinase for hydrolysing the glucoside. Crushing the tubers releases these compounds and produces acetone cyanohydrin, which is potentially lethally toxic. Acetone cyanohydrin is classified as an extremely hazardous substance in the U.S. Emergency Planning and Community Right-to- Know Act. The principal hazards of acetone cyanohydrin arise from its ready decomposition on contact with water, which releases highly toxic hydrogen cyanide.
Chemical properties
clear yellowish liquid
Chemical properties
cetone cyanohydrin is a colorless, fl ammable liquid with a faint odor of bitter almond. It is an organic compound used in the production of methyl methacrylate, the monomer of the transparent plastic polymethyl methacrylate (PMMA), also known as acrylic. It is incompatible with sulfuric acid and caustics. Acetone cyanohydrin readily undergoes decomposition by water to form hydrogen cyanide and acetone.
Chemical properties
Acetone cyanohydrin is a flammable, colorless to light yellow liquid. Almond-like odor.
The Uses of Acetone cyanohydrin
Acetone cyanohydrin is used to make methyl methacrylate; in the production of pharmaceuticals, foaming agents, and insecticides; and to produce polymerization initiators.
The Uses of Acetone cyanohydrin
In preparative organic chemistry for transcyanohydrination, such as preparing the 17-monocyanohydrin of a 3,17-diketo steroid by hydrogen cyanide exchange with the reagent, e.g. Ercoli, de Ruggieri, J. Am. Chem. Soc. 75, 650 (1953).
The Uses of Acetone cyanohydrin
Acetone cyanohydrin is used as a raw material for insecticides manufacture and also to produce ethyl α-hydroxyisobutryate, a pharmaceutical intermediate. It has been used as a complexing agent for metals refining and separation.
Production Methods
Acetone cyanohydrin is formed by adding acetone to sodium or potassium cyanide in water and treating with H2S04 or by reaction of acetone with hydrogen cyanide (HSDB 1988; Windholz et al 1983). Technical acetone cyanohydrin may contain as much as 0.2% free hydrogen cyanide. U.S. production in 1984 is estimated at 546,000 tons (HSDB 1988).
Production Methods
Acetone cyanohydrin is manufactured by the direct reaction of hydrogen cyanide with acetone, catalyzed by base, generally in a continuous process.
Definition
ChEBI: 2-hydroxy-2-methylpropanenitrile is a cyanohydrin.
General Description
A colorless liquid. Flash point 165°F. Lethal by inhalation and highly toxic or lethal by skin absorption. Density 7.8 lb / gal (less dense than water). Vapors heavier than air. Produces toxic oxides of nitrogen during combustion.
Reactivity Profile
ACETONE CYANOHYDRIN readily decomposes to acetone and poisonous hydrogen cyanide gas on contact with water, acids (sulfuric acid) or when exposed to heat. Should be kept cool and slightly acidic (pH 4-5) [Sax, 2nd ed., 1965, p. 388]. Slowly dissociates to acetone, a flammable liquid, and hydrogen cyanide, a flammable poisonous gas, under normal storage and transportation conditions. Rate of dissociation increased by contact with alkalis and/or heat.
Health Hazard
Acetone cyanohydrin is considered very hazardous and should only be handled under conditions that prevent any inhalation of vapor or skin contact. May be slightly irritating to skin and mucous membranes.
Health Hazard
Acetone cyanohydrin is extremely toxic. Exposures to acetone cyanohydrin cause adverse health effects. The symptoms of toxicity include, but are not limited to, irritation to the eyes, skin, respiratory system, dizziness, lassitude (weakness, exhaustion), headache, con- fusion, pulmonary edema, asphyxia convulsions, liver, kidney injury, pulmonary edema, and asphyxia. The target organs include the eyes, skin, respiratory system, central nervous system (CNS), cardiovascular system, liver, kidneys, and the gastrointestinal tract.
Health Hazard
Toxic effects of acetone cyanohydrin have been studied by Sunderman and Kincaid (1953), Lang and Stintzy (1960), and Thiess (1969). Skin contact with acetone cyanohydrin caused nausea, vomiting, dyspnea, cardiac palpitation, convulsion, cyanosis, coma, and death. Oral ingestion of an unknown amount of acetone cyanohydrin caused death 12 h post exposure.
Health Hazard
Acetone cyanohydrin is a highly poisonous compound. Although the acute toxicity is lower than that associated with hydrogen cyanide, the toxic effects are similar to those of the latter compound. The toxic routes are inhalation, ingestion, and skin contact.
Inhalation of its vapors at high concentrations can cause instantaneous loss of consciousness and death. Exposure to 63 ppm for 4 hours was lethal to rats. The oral toxicity of this compound in test animals was found to be high with LD50 values falling between 10 and 20 mg/kg.
LD50 value, oral (mice): 14 mg/kg
It is a mild irritant to the skin but moderately toxic by skin absorption. There is no report of its teratogenic and carcinogenic actions in humans or animals.
Fire Hazard
Too dangerous to health to expose fire fighters; a few whiffs of vapor could cause death; vapor or liquid could be fatal on penetrating normal protective clothing. Vapor forms explosive mixture with air. Decomposes when heated to 248F or at lower temperature under alkaline conditions, emitting highly toxic hydrogen cyanide. May react violently with water. Contact with sulfuric acid may cause Acetone cyanohydrin to explode.
Industrial uses
Actone cyanohydrin is used primarily in the preparation of methyl methacrylate, which is polymerized to form various plastics, including plexiglas, and as intermediate for resins (Hawley 1981). It also is employed for the manufacture of insecticides; to produce oc-hydroxy-isobutyrate, a pharmaceutical intermediate; as a complexing agent for metals; and as a reagent for chemical synthesis.
Safety Profile
Poison by ingestion, skin contact, inhalation, intraperitoneal, and subcutaneous routes. Readily decomposes to HCN and acetone. Keep cool and do not store for long periods. Combustible when exposed to heat or flame. To fight fire, use CO2, dry chemical, alcohol foam. Vigorous reaction with H2SO4. When heated to decomposition it emits toxic fumes of CN-.
Potential Exposure
Used in the manufacture of insecticides and making other chemicals, such as methyl methacrylate.
Metabolism
Acetone cyanohydrin is readily absorbed by all routes (Hartung 1981). Shkodich (1966) found no evidence of cumulative effects of acetone cyanohydrin in white mice or albino rats over a period of 20 d in which daily doses of one-fifth of the LD50s were given. Rapid detoxication and excretion were presumed to occur. Acetone cyanohydrin may be largely metabolized to yield free cyanide (H?rtung 1981).
Shipping
UN1541 Acetone cyanohydrin, stabilized, Hazard Class: 6.1; Labels: 6.1-Poison Inhalation Hazard, Inhalation Hazard Zone B.
Purification Methods
Dry the cyanohydrin with Na2SO4, and distil it as rapidly as possible under vacuum to avoid decomposition. Discard fractions boiling below 78-82o/15mm. Store it in the dark. USE AN EFFICIENT FUME HOOD as HCN (POISONOUS) is always present. [Cox & Stormont Org Synth Coll.Vol. II 7 1940, Beilstein 3 H 316, 3 IV 785.]
Incompatibilities
May form explosive mixture with air. Not compatible with strong reducers, strong bases; strong oxidizers, and strong acids, such as hydrochloric, sulfuric (explosive), and nitric. Contact with strong acid and strong bases may cause explosions. Slowly decomposes to acetone and hydrogen cyanide gas at room temperatures; rate is accelerated by an increase in pH, contact with water, or temperature.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Add with stirring to strong alkaline calcium hypochlorite solution. Alternatively dissolve in flammable solvent and burn in incinerator with afterburner and scrubber.
Properties of Acetone cyanohydrin
| Melting point: | -19 °C (lit.) |
| Boiling point: | 82 °C/23 mmHg (lit.) |
| Density | 0.932 g/mL at 25 °C (lit.) |
| vapor pressure | 0.3 psi ( 20 °C) |
| refractive index | n |
| Flash point: | 147 °F |
| storage temp. | Refrigerator |
| solubility | very soluble in water, alcohol, and ether; slightly soluble in petroleum
ether |
| form | Liquid |
| pka | 11.45±0.29(Predicted) |
| color | Clear colorless to yellow |
| Water Solubility | miscible |
| Merck | 13,68 |
| Exposure limits | Ceiling 4 mg (1.2 ppm)/m3/15 min (NIOSH). Zholdakova et al. (1995) have recommended a maximum permissible concentration of 0.035 mg/L in water as cyanide for acetone cyanohydrin or in combination with other cyanides. |
| CAS DataBase Reference | 75-86-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Propanenitrile, 2-hydroxy-2-methyl-(75-86-5) |
| EPA Substance Registry System | Acetone cyanohydrin (75-86-5) |
Safety information for Acetone cyanohydrin
Computed Descriptors for Acetone cyanohydrin
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Acetone cyanohydrin CAS 75-86-5View Details
Acetone cyanohydrin CAS 75-86-5View Details
75-86-5 -
 Acetone cyanohydrin CAS 75-86-5View Details
Acetone cyanohydrin CAS 75-86-5View Details
75-86-5 -
 2-Hydroxy-2-Methyl-Propanenitrile CAS 75-86-5View Details
2-Hydroxy-2-Methyl-Propanenitrile CAS 75-86-5View Details
75-86-5 -
 Acetone cyanohydrin CAS 75-86-5View Details
Acetone cyanohydrin CAS 75-86-5View Details
75-86-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1