2,2'-Azobis(2-methylpropionitrile)
Synonym(s):AIBN;Azobisisobutyronitrile;Free radical initiator;radical initiator;AIBN solution
- CAS NO.:78-67-1
- Empirical Formula: C8H12N4
- Molecular Weight: 164.21
- MDL number: MFCD00013808
- EINECS: 201-132-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
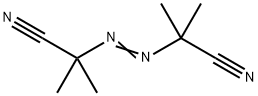
What is 2,2'-Azobis(2-methylpropionitrile)?
Description
2,2''-Azobis(isobutyronitrile), or AIBN, is a commonly used initiator for free-radical reactions, including many polymerizations. Its preparation was first reported by Thiele and Heuser in 1896, and it has been used in polymerization since at least the 1940s. Its downsides are high toxicity (it decomposes to HCN in vivo), flammability, and the explosivity of its acetone solutions.
Description
Azobisisobutylonitrile is a white crystallinecompound. Molecular weight= 164.21; Specific gravity(H2O:1)= 1.11 at 25℃; Boiling point= decomposes;Freezing/Melting point= 99°103℃ (decomposes);Autoignition temperature= 63℃. NFPA 704 M HazardIdentification (based on NFPA-704M Rating System):Health 3, Flammability 2, Reactivity 2. Insoluble in water.
Chemical properties
Azobisisobutylonitrile is a white crystalline compound.
The Uses of 2,2'-Azobis(2-methylpropionitrile)
2,2'-Azobis(2-methylpropionitrile) (AIBN) is a foaming agent and inhibitor in plastic and elastomer materials. It is a radical initiator. AIBN solution can be used to initiate radical-induced reactions, specifically free-radical polymerizations. It can be used in:
Synthesis of styrene-vinyl pyridine diblock copolymers by reversible addition-fragmentation chain transfer (RAFT) polymerization.
Preparation of silicon oxycarbide glasses.
Synthesis of poly [N-(p-vinyl benzyl) phthalimide] for preparing titanium dioxide composites for electrophoretic displays.
Used as an initiator in synthesising highly cross-linked Poly(divinylbenzene) (PDVB) polymers.
Used as an initiator in the polymerization process of 2-hydroxyethyl methacrylate (HEMA).
Definition
ChEBI: (E)-Azobis(isobutyronitrile) is an azo compound.
General Description
Insoluble in water and denser than water. Moderately toxic by ingestion. Readily ignited by sparks or flames. Burns intensely and persistently. Toxic oxides of nitrogen produced during combustion. Used as a catalyst, in vinyl polymerizations and a blowing agent for plastics.
Air & Water Reactions
Dust may form an explosive mixture in air. Insoluble in water.
Reactivity Profile
Self-decomposition or self-ignition may be triggered by heat, chemical reaction, friction or impact. Self-accelerating decomposition may occur if the specific control temperature is not maintained. These materials are particularly sensitive to temperature rises. 2,2'-Azobis(2-methylpropionitrile) is an azo compound. Azo, diazo, azido compounds can detonate. This applies in particular to organic azides that have been sensitized by the addition of metal salts or strong acids. Toxic gases are formed by mixing materials of this class with acids, aldehydes, amides, carbamates, cyanides, inorganic fluorides, halogenated organics, isocyanates, ketones, metals, nitrides, peroxides, phenols, epoxides, acyl halides, and strong oxidizing or reducing agents. Flammable gases are formed by mixing materials in this group with alkali metals. Explosive combination can occur with strong oxidizing agents, metal salts, peroxides, and sulfides.
Hazard
Toxic by ingestion.
Flammability and Explosibility
Not classified
Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion. Easily oxidized, unstable. Violent exothermic decomposition when heated. Solution in acetone may decompose explosively. Explodes when heated with heptane. When heated to decomposition it emits toxic fumes of NO, and CN-. See also NITRILES. A free-radcal generator.
Potential Exposure
Azobisisobutylonitrile is both a nitrile and azo compound. Used as a polymerization initiator, free radical generator (or initiator); as a catalyst in vinyl polymerizations; as a blowing agent for elastomers and plastics.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Note: Use amyl nitrate capsules if symptoms develop. Allarea employees should be trained regularly in emergencymeasures for cyanide poisoning and in CPR. A cyanide antidote kit should be kept in the immediate work area and mustbe rapidly available. Kit ingredients should be replaced every1°2 years to ensure freshness. Persons trained in the use ofthis kit, oxygen use, and CPR must be quickly available.
Storage
Color Code—Red Stripe: Flammability Hazard: Donot store in the same area as other flammable materials. Priorto working with this chemical you should be trained on itsproper handling and storage. Azodiisobutyronitrile must bestored to avoid contact with acetone, lithium, aluminumhydride, and water, since violent reactions occur. Azodiisobutyronitrile is self-reactive and will explode at elevated temperatures. It should be stored under nitrogen, dry ice, or ice.Sources of ignition, such as smoking and open flames, areprohibited where this chemical is used, handled, or stored in amanner that could create a potential fire or explosion hazard.
Shipping
UN3234 Self-reactive solid type C, temperature controlled materials, Hazard Class: 4.1; Labels: 4.1- Flammable solid, Technical Name Required.
Incompatibilities
Flammable; dust may form explosive mixture with air. Unstable and easily oxidized material; keep away from oxidizers, strong acids. Keep at temperature not ≧30° C (this may vary by manufacturer). Risk of explosion from heat, shock, friction. Warming causes production of tetramethylsuccinonitrile and cyanide fumes. Keep away from acetone and other ketones, alcohols, lithium, aluminum, aldehydes, and hydrocarbons, such as heptane. Azo compounds can detonate. This applies in particular to organic azides that have been sensitized by the addition of metal salts or strong acids. Toxic gases are formed by mixing materials of this class with acids, aldehydes, amides, carbamates, cyanides, inorganic fluorides, halogenated organics, isocyanates, ketones, metals, nitrides, peroxides, phenols, epoxides, acyl halides, and strong oxidizing or reducing agents. Flammable gases are formed by mixing materials in this group with alkali metals. Explosive combination can occur with strong oxidizing agents, metal salts, peroxides, and sulfides. This chemical is sensitive to prolonged exposure to heat. This chemical is incompatible with strong oxidizing agents.
Properties of 2,2'-Azobis(2-methylpropionitrile)
| Melting point: | 102-104 °C (dec.)(lit.) |
| Boiling point: | 281.68°C (rough estimate) |
| Density | 1.11 |
| vapor pressure | 0.81Pa at 24.85℃ |
| refractive index | n20/D1.495 |
| Flash point: | 4℃ |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| appearance | White crystals |
| color | Crystals from EtOH |
| Odor | odorless |
| Water Solubility | Insoluble |
| Merck | 13,920 |
| BRN | 1708400 |
| Stability: | Stability Flammable solid. Shock sensitive. Thermally unstable. May be explosive in combination with acetone or heptane. Incompatible with oxidizing agents. |
| CAS DataBase Reference | 78-67-1(CAS DataBase Reference) |
| NIST Chemistry Reference | 2,2'-Azo-bis-isobutyronitrile(78-67-1) |
| EPA Substance Registry System | Azobis(isobutyronitrile) (78-67-1) |
Safety information for 2,2'-Azobis(2-methylpropionitrile)
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H242:Self-reactive substances and mixtures; and Organic peroxides H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P235:Keep cool. P273:Avoid release to the environment. P370+P378:In case of fire: Use … for extinction. P403:Store in a well-ventilated place. |
Computed Descriptors for 2,2'-Azobis(2-methylpropionitrile)
| InChIKey | OZAIFHULBGXAKX-VAWYXSNFSA-N |
2,2'-Azobis(2-methylpropionitrile) manufacturer
JSK Chemicals
NSR Amino Organics
Krystal Tech
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 78-67-1 AIBN (Azobisisobutyronitrile) 99%View Details
78-67-1 AIBN (Azobisisobutyronitrile) 99%View Details
78-67-1 -
 Azobisisobutyronitrile CAS 78-67-1View Details
Azobisisobutyronitrile CAS 78-67-1View Details
78-67-1 -
 AIBN CAS 78-67-1View Details
AIBN CAS 78-67-1View Details
78-67-1 -
 a,a'-AZOISOBUTYRONITRILE For Synthesis CAS 78-67-1View Details
a,a'-AZOISOBUTYRONITRILE For Synthesis CAS 78-67-1View Details
78-67-1 -
 2,2′-Azobis(2-methylpropionitrile) solution CAS 78-67-1View Details
2,2′-Azobis(2-methylpropionitrile) solution CAS 78-67-1View Details
78-67-1 -
 Azobisisobutyronitrile CAS 78-67-1View Details
Azobisisobutyronitrile CAS 78-67-1View Details
78-67-1 -
 Azobisisobutyronitrile chemical CAS Number: 78-67-1View Details
Azobisisobutyronitrile chemical CAS Number: 78-67-1View Details
78-67-1 -
 Reagent Grade Powder A,A-Azoisobutyronitrile 98% For SynthesisView Details
Reagent Grade Powder A,A-Azoisobutyronitrile 98% For SynthesisView Details
78-67-1
