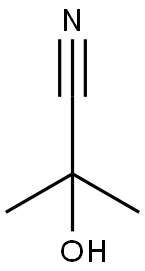
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Acetone cyanohydrin, stabilized appears as a colorless liquid. Flash point 165 °F. Lethal by inhalation and highly toxic or lethal by skin absorption. Density 7.8 lb / gal (less dense than water). Vapors heavier than air. Produces toxic oxides of nitrogen during combustion. |
|---|---|
| Color/Form | Colorless liquid |
| Odor | Distinct strong cyanide odor |
| Boiling Point | 180 °F at 23 mmHg (EPA, 1998) |
| Melting Point | -2.2 °F (EPA, 1998) |
| Flash Point | 165 °F (EPA, 1998) |
| Solubility | greater than or equal to 100 mg/mL at 68 °F (NTP, 1992) |
| Density | 0.9267 at 77 °F (EPA, 1998) - Less dense than water; will float |
| Vapor Density | 2.93 (EPA, 1998) - Heavier than air; will sink (Relative to Air) |
| Vapor Pressure | 0.8 mmHg at 68 °F (EPA, 1998) |
| Autoignition Temperature | 1270 °F (USCG, 1999) |
| Decomposition | When heated to decomposition it emits toxic fumes of /hydrogen cyanide/. |
| Heat of Combustion | -2.2391X10+9 J/kmol /Estimated, Std Net Heat of Comb/ |
| Odor Threshold | Odor Threshold Low: 3.0 [mmHg] Odor threshold (recognition) |
| Refractive Index | Index of refraction: 1.3992 at 20 °C/D |
| Relative Evaporation Rate | < 1 (water = 1.0) |
| Chemical Classes | Nitrogen Compounds -> Nitriles |
COMPUTED DESCRIPTORS
| Molecular Weight | 85.10 g/mol |
|---|---|
| XLogP3 | -0.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 85.052763847 g/mol |
| Monoisotopic Mass | 85.052763847 g/mol |
| Topological Polar Surface Area | 44 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 87 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Acetone cyanohydrin, stabilized appears as a colorless liquid. Flash point 165 °F. Lethal by inhalation and highly toxic or lethal by skin absorption. Density 7.8 lb / gal (less dense than water). Vapors heavier than air. Produces toxic oxides of nitrogen during combustion.