Acephate
Synonym(s):O,S-Dimethyl N-acetylphosphoramidothioate
- CAS NO.:30560-19-1
- Empirical Formula: C4H10NO3PS
- Molecular Weight: 183.17
- MDL number: MFCD00055361
- EINECS: 250-241-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
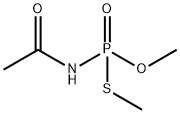
What is Acephate?
Description
Acephate is an organophosphate foliar spray insecticide of moderate persistence with residual systemic activity. It is a contact and systemic insecticide and very effective against a large number of crop pests such as alfalfa looper, aphids, armyworms, bagworms, bean leaf beetle, bean leafroller, black grass bugs, bollworm, budworm, and cabbage looper. Acephate is a colourless to white solid organophosphate insecticide. Exposures to acephate cause poisoning to animals and humans. Acephate inhibits acetylcholine esterase (AchE), the essential nervous system enzyme, and causes characteristic organophosphate poisoning. The symptoms of toxicity include, but are not limited to, headache, nervousness, blurred vision, weakness, nausea, fatigue, stomach cramps, diarrhoea, difficulty in breathing, chest pain, sweating, pinpoint pupils, tearing, salivation, clear nasal discharge and sputum, vomiting, muscle twitching, muscle weakness, and, in severe poisoning, convulsions, respiratory depression, coma, and death. Acephate causes cholinesterase inhibition leading to overstimulation, respiratory paralysis, and death. The U.S. EPA classified acephate as Group C, meaning a possible human carcinogen, and therefore requires judicious handling and management.
Chemical properties
White Solid
Chemical properties
Acephate is an organophosphate foliar spray insecticide of moderate persistence with residual systemic activity. It is a contact and systemic insecticide and very effective against a large number of crop pests, such as alfalfa looper, aphids, armyworms, bagworms, bean leafbeetle, bean leafroller, blackgrass bugs, bollworm, budworm, and cabbage looper.
Chemical properties
Colorless crystalline solid (80% or more pure) or white powder (technical). Has an odor of rotten cabbage or mercaptans (like sulfur).
The Uses of Acephate
Acephate is used to control a wide range of sucking and chewing insects in a large number of crops.
The Uses of Acephate
Acephate is an organophosphate foliar spray insecticide of moderate persistence with residual systemic activity. It is a contact and systemic insecticide and very effective against a large number of crop pests, such as alfalfa looper, aphids, armyworms, bagworms, bean leafbeetle, bean leafroller, blackgrass bugs, bollworm, budworm, and cabbage looper.
The Uses of Acephate
Contact and systemic insecticide for control of sucking and chewing insects in cotton, ornamentals, forestry, tobacco, fruits, vegetables and other crops
What are the applications of Application
Acephate is an inhibitor of acetylcholinesterase (AChE)
Definition
ChEBI: A phosphoramide that is methamidophos in which one of the hydrogens is replaced by an acetyl group.
General Description
A white solid. Used as a contact and systemic insecticide.
Air & Water Reactions
Soluble in water.
Reactivity Profile
A thiophosphate ester. Organothiophosphates are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides.
Health Hazard
Acephate is a colorless to white, solid organophosphate insecticide. Exposures to acephate cause poisoning to animals and humans. Acephate inhibits acetylcholine esterase (AchE), the essential nervous system enzyme, and causes characteristic organophosphate poi- soning. The symptoms of toxicity include, but are not limited to, headache, nervousness, blurred vision, weakness, nausea, fatigue, stomach cramps, diarrhea, diffi culty breathing, chest pain, sweating, pin-point pupils, tearing, salivation, clear nasal discharge and spu- tum, vomiting, muscle twitching, muscle weakness, and in severe poisonings, convulsions, respiratory depression, coma, and death. Acephate causes cholinesterase inhibition lead- ing to overstimulation, respiratory paralysis, and death.
Agricultural Uses
Insecticide: Acephate is a general use contact and systemic insecticide. Not approved for use in EU countries. Actively registered in the U.S., homeowner use for lawns is discontinued except for treatment of fire ant mounds. Other indoor treatment has been discontinued. Used on green and lima beans, Brussels sprouts, cauliflower, celery, cotton, cottonseed, cranberries, head lettuce, macadamia nuts, peanuts, bell-and non-bell peppers, peppermint, spearmint, tobacco, and soybeans (Special Local Need Registration required in Mississippi and Texas only). Also used to control cockroach (spot treatment only) in residential and industrial buildings and insect control in forests, and on ornamental plants and to target armyworms, aphids, beetles, bollworms, borers, budworms, cankerworms, crickets, cutworms, fire ants, fleas, grasshoppers, leafhoppers, loopers, mealybugs, mites, moths, roaches, spiders, thirps, wasps, weevils, whiteflies, etc.
Trade name
ACECAP SYSTEMIC INSECTICIDE IMPLANTS®; ACEFAL 75 PS®; ACEHERO®; ACEPHATE 97 EG®; ACEPHATE 75SP®; ACEPHATE PCO SP INSECTICIDE®; ACESUL®; ACE-TOX®; ACHERO®; ACIFAT®; ADDRESS®; AIMTHENE®; AMCOTHENE®; ASATAF®; ASIFY®; ATTACK®; CHEVRON RE 12420®; CLEAN CROP ACEPHATE 80 DF SEED PROTECTORANT®; DREXEL ACEPHATE 75 WSP®; DREXEL ACEPHATE PCO SP INSECTICIDE®; FATEL®; FORPHATE®, FORWARD®; KITRON®; KORANDA® (acephate + fenvelerate); LANCER®; ORCEPHATE®; ORTHENE®; ORTHENE 755®; Acephate 3 ORTHO 12420®; ORTRAN®; ORTRIL®; PACE®; PAYLOAD®; PILARTHENE®; PINPOINT®; PRECISE ACEPHATE®; RACET®; RE 12420®; SAPHATE®; 75 SP®; VALENT ORTHENE TECHNICAL®; VEGFRU TARGET®
Biological Activity
Anticholinesterase insecticide that produces cholinotoxicity. Displays weak inhibition of rat acetylcholinesterase (AChE) but potently inhibits cockroach AChE.
Safety Profile
Poison by ingestion. Moderately toxic by skin contact and inhalation. Human mutation data reported. When heated to decomposition it emits very toxic fumes of NOx, POx, and SOx. See also ESTERS.
Potential Exposure
Acephate is a general use contact and systemic insecticide. Banned in the EU for use as a biocide and agricultural insecticide. Used on green- and limabeans, Brussels sprouts, cauliflower, celery, cotton, cottonseed, cranberries, head lettuce, macadamia nuts, peanuts, bell-and nonbell peppers, peppermint, spearmint, tobacco, and soybeans (Special Local Need Registration required in Mississippi and Texas only). Also used to control cockroach (spot treatment only) in residential and industrial buildings and insect control in forests, and on ornamental plants and to target armyworms, aphids, beetles, bollworms, borers, budworms, cankerworms, crickets, cutworms, fire ants, fleas, grasshoppers, leafhoppers, loopers, mealybugs, mites, moths, roaches, spiders, thirps, wasps, weevils, whiteflies, etc. banned for use in the EU.
Carcinogenicity
Acephate showed no evidence of carcinogenicity among rats given diets with 0, 5, 50, or 700 ppm (equivalent to about 0, 0.25, 2.5, and 35.0 mg/kg/ day, respectively) for 28 months .
Environmental Fate
Soil. In aerobic and anaerobic soils, methamidophos and carbon dioxide were identified
as the major soil metabolites (Hartley and Kidd, 1987). The estimated half-life in soil is
3 days (Wauchope, 1988)
Plant. Acephate is quickly absorbed, translocated and transformed in pine seedlings
(Werner, 1974) and cotton plants (Bull, 1979). The chemical was metabolized via cleavage
of the amide bond to form methamidophos (O,S-dimethyl phosphoramidothioat
Chemical/Physical. Emits toxic fumes of phosphorus, nitrogen and sulfur oxides when
heated to decomposition (Sax and Lewis, 1987)
Metabolic pathway
Acephate is a systemic insecticide with a very favourable mammalian toxicity. The initial reaction of the biotransformation of acephate is by hydrolysis to the active acetylcholinesterase inlubitor methamidophos. In every case where the metabolism of acephate has been studied in biological systems, the production of methamidophos has been demonstrated; however, the amount of this presumed active metabolite varies greatly from organism to organism. In mammals, which are relatively insensitive to the insecticide, the primary route of stage I metabolism is mainly degradative via O-demethylation to give des-O-methylacephate. Further metabolism in mammals and birds leads to incorporation of the molecule into proteins, carbohydrates and lipids as well as to excretion. Conjugates have not been identified.
Shipping
UN2783 Organophosphorus pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN3018 Organophosphorus pesticides, liquid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous material
Degradation
Acephate is relatively stable to hydrolysis, with DT50s of 60 hours at pH 9
and 710 hours at pH 3 and 40 °C (PM).
Chukwudebe et al. (1984) studied the breakdown of acephate in 0.1M
Tris-HCl buffer at pH 7.2, 8.1 and 8.6 at 37 °C. The hydrolysis products
were analysed by GLC with FID for acephate and methamidophos and
TLC separation and GLC analysis for the other products. Authenticated
standards [methamidophos (2), des-O-methylacephate (3), des-S-methylacephate
(4) and O,S-dimethyl phosphorothioate (5)] were used to
calibrate the analysis. The major metabolite at all pH values was O,S-dimethyl
phosphorothioate (5). This reached a maximum concentration
of 40-55% in 1-3 days depending on pH. Its concentration then declined
as it was itself hydrolysed. The hydrolysis of acephate was strongly
biphasic, with hydrolysis becoming very slow after about 2 days. The
amounts of methamidophos (2) (3%) and des-S-methylacephate (4) (10%)
produced after 7 days were minor. Major and minor routes for the
hydrolysis of methamidophos are shown in Scheme 1.
Toxicity evaluation
Although methamidophos is highly toxic to mammals (LD50 mice = 27 mg/kg), the acetylation causes a dramatic decrease in the mammalian toxicity; the acute oral LD50 values of acephate for mice and rats are 361 and 945 mg/kg, respectively. Inhalation LC50 is >15 mg/L air. In 2 years of feeding trials, dogs showed depression of cholinesterase at 100 mg/kg diet (5 mg/kg/d) (maximum dose level). ADI is 0.03 mg/kg.
Incompatibilities
May react with strong oxidizers such as chlorates, peroxides, nitrates, etc. In the presence of strong reducing agents such as hydrides, organophosphates form highly toxic and flammable phosphine gas. Contact with oxidizers can cause the release of toxic oxides of phosphorus. Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water, and a salt that may be harmful. Incompatible with arsenic compounds(releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides (releasing heat, toxic and possibly flammable gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur).
Waste Disposal
Alkaline hydrolysis or incineration. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Containers must be disposed of properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Properties of Acephate
| Melting point: | 93°C |
| Density | 1.35 |
| vapor pressure | 2.26 x 10-4 Pa (24 °C) |
| Flash point: | 2 °C |
| storage temp. | APPROX 4°C
|
| solubility | Chloroform: Soluble; DMSO: Soluble; Water: Soluble |
| pka | 11.00±0.46(Predicted) |
| form | solid |
| color | White to yellow |
| Water Solubility | readily soluble |
| Merck | 13,32 |
| BRN | 1936365 |
| InChI | InChI=1S/C4H10NO3PS/c1-4(6)5-9(7,8-2)10-3/h1-3H3,(H,5,6,7) |
| NIST Chemistry Reference | Acephate(30560-19-1) |
| EPA Substance Registry System | Acephate (30560-19-1) |
Safety information for Acephate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. |
Computed Descriptors for Acephate
| InChIKey | YASYVMFAVPKPKE-UHFFFAOYSA-N |
| SMILES | P(NC(C)=O)(SC)(OC)=O |
Acephate manufacturer
Mangalore Chemicals And Fertilizers Limited
HPM Chemicals And Fertilizers Ltd
Super Crop Safe Limited
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran





You may like
-
 Acephate 99%View Details
Acephate 99%View Details
30560-19-1 -
 Acephate 30560-19-1 98%View Details
Acephate 30560-19-1 98%View Details
30560-19-1 -
 Acephate 30560-19-1 98%View Details
Acephate 30560-19-1 98%View Details
30560-19-1 -
 30560-19-1 Acephate 98%View Details
30560-19-1 Acephate 98%View Details
30560-19-1 -
 30560-19-1 98%View Details
30560-19-1 98%View Details
30560-19-1 -
 30560-19-1 Acephate 98%View Details
30560-19-1 Acephate 98%View Details
30560-19-1 -
 Orthene CAS 30560-19-1View Details
Orthene CAS 30560-19-1View Details
30560-19-1 -
 Acephate CAS 30560-19-1View Details
Acephate CAS 30560-19-1View Details
30560-19-1