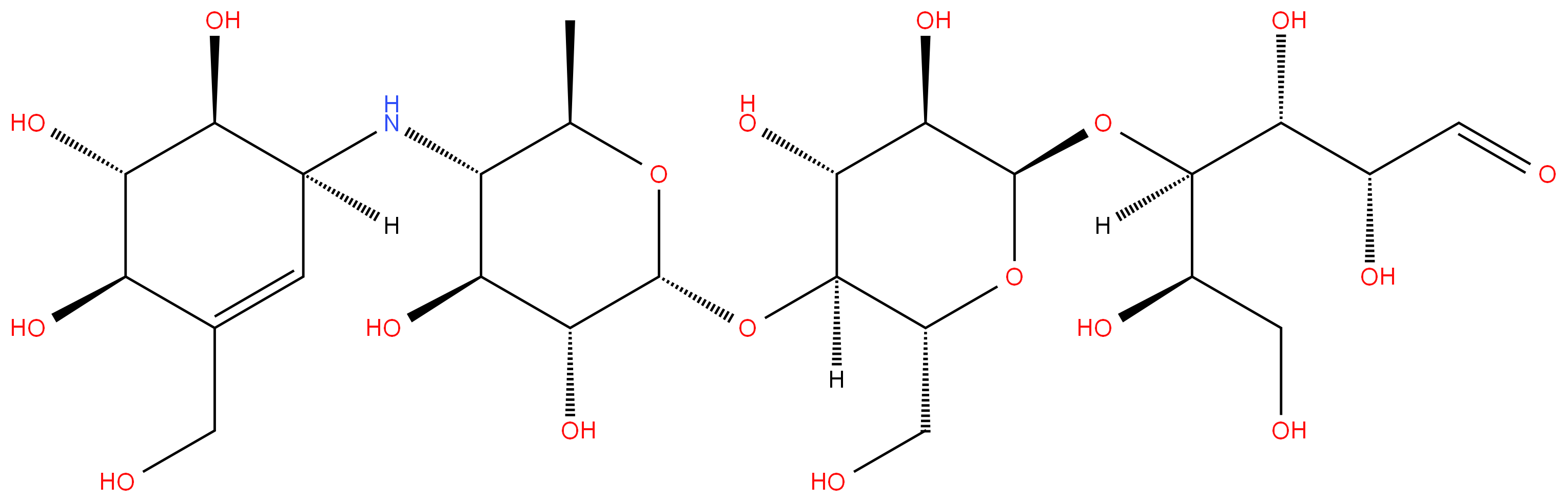
Acarbose
Synonym(s):4",6"-Dideoxy-4"-([1S]-[1,4,6/5]-4,5,6-trihydroxy-3-hydroxymethyl-2-yclohexenylamino)-maltotriose;Acarbose
- CAS NO.:56180-94-0
- Empirical Formula: C25H43NO18
- Molecular Weight: 645.61
- MDL number: MFCD00869592
- EINECS: 260-030-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
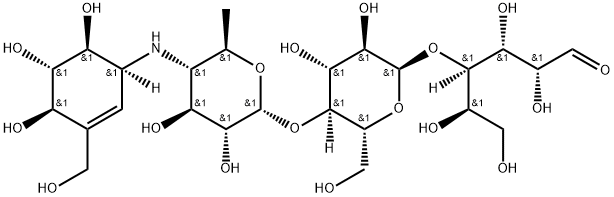
What is Acarbose?
Absorption
The oral bioavailability of acarbose is extremely minimal, with less than 1-2% of orally administered parent drug reaching the systemic circulation. Despite this, approximately 35% of the total radioactivity from a radiolabeled and orally administered dose of acarbose reaches the systemic circulation, with peak plasma radioactivity occurring 14-24 hours after dosing - this delay is likely reflective of metabolite absorption rather than absorption of the parent drug. As acarbose is intended to work within the gut, its minimal degree of oral bioavailability is therapeutically desirable.
Toxicity
The symptoms of acarbose overdose are likely to be consistent with its adverse effect profile and may therefore include significant gastrointestinal (GI) symptoms (flatulence, distension, etc), although an overdose on an empty stomach (i.e. when not co-administered with food) is less likely to result in these GI symptoms. In the event of an overdose, patients should be instructed to avoid carbohydrate-containing foods for 4-6 hours following administration as these can precipitate the aforementioned GI symptoms.
Description
Acarbose, a complex oligosaccharide isolated from Actinoplanes, is reportedly useful as an adjuvant therapy in diabetes. By inhibiting alpha-glucosidase, acarbose delays carbohydrate metabolism in the gastrointestinal tract and modulates changes in food induced blood sugar levels.
Description
Acarbose is a modified tetrasaccharide that is used to treat type 2 diabetes and sometimes prediabetes. The images show the right-hand saccharide in its aldehyde form; but in solution, it cyclizes to form a pyranose.
Acarbose is an α-glucosidase inhibitor that prevents the enzymatic release of glucose from larger carbohydrates in the gut. This process aids in the treatment of diabetes by reducing the amount of sugar absorbed in the intestinal tract.
In the mid-1970s, six researchers at Bayer AG (Leverkusen, West Germany) were awarded German and US patents for the synthesis of acarbose and similar compounds. (The US patent is simply titled “Amino Sugar Derivatives”.) The description of the invention states that the compounds could be used to treat diabetes, adiposity, and hyperlipidemia; but no disease treatment is mentioned in the claims.
Acarbose, now a generic drug, is marketed under several tradenames worldwide. It is prescribed rarely in the United States, however, because it frequently causes diarrhea and flatulence. In contrast, it is very popular in China because it is inexpensive and the side effects are minimal. The difference is attributed to Chinese and other Asian diets, which contain considerably more carbohydrates than Western diets.
In May, chemist Daniel C. Whitehead, microbiologist Kristi Whitehead, and colleagues at Clemson University (SC) demonstrated that acarbose might be used to modify bacterial populations in the human microbiome. Specifically, in lab-grown cultures, the drug prevented the growth of Bacteroides species, which consume potato starch and the food additive fungal pullulan.
Although it is yet to be shown what effect acarbose has on the actual microbiome, Daniel Whitehead says, “This work is really a proof of concept that a small molecule can arrest the starch utilization system.”?
Chemical properties
Off-White Solid
Originator
Bayer AG (Germany)
The Uses of Acarbose
Acarbose is pseudo-oligosaccharide with a terminal C7-cyclitol patented in 1975 by Bayer. Acarbose is a component of the amylostatin complex produced by a species of Actinoplanes and Streptomyces. Acarbose acts as a potent inhibitor of alpha-glucosidases and saccharases. Since 1990, acarbose has been used therapeutically for the treatment of type 2 diabetes.
The Uses of Acarbose
antipsychotic: 5HT antagonist, dopamine antagonist, H1-antihistamine, alpha adrenergic blocker
The Uses of Acarbose
A reversible alpha-glucosidase inhibitorAcarbose is used as a reversible alfa-glucosidase inhibitor. It acts as an anti-diabetic drug, which finds useful application to treat type 2 diabetes mellitus and prediabetes. It is also employed for Hepatitis. It also acts as a beta glucosidase inhibitor, antihyperlipidaemia and antiobesity.
Indications
Acarbose is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Definition
ChEBI: A tetrasaccharide derivative consisting of a dideoxy-4-{[4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-en-1-yl C7 cyclitol moiety [called valienol (or valienamine)] linked via nitrogen to isomaltotriose.
What are the applications of Application
Acarbose is an α-glucosidase inhibitor that can function as an anti-diabetic by preventing absorption of sucrose and maltose.
Background
Acarbose is a complex oligosaccharide that acts as an inhibitor of several enzymes responsible for the breakdown of complex carbohydrates in the intestines. It inhibits both pancreatic alpha-amylase and membrane-bound alpha-glucosidases - including intestinal glucoamylase, sucrase, maltase, and isomaltase - which are responsible for the metabolism of complex starches and oligo-, tri-, and disaccharides into absorbable simple sugars. By inhibiting the activity of these enzymes, acarbose limits the absorption of dietary carbohydrates and the subsequent postprandial increase in blood glucose and insulin levels. Acarbose is therefore used in conjunction with diet, exercise, and other pharmacotherapies for the management of blood sugar levels in patients with type 2 diabetes.
Acarbose is one of only two approved alpha-glucosidase inhibitors (the other being miglitol), receiving its first FDA approval in 1995 under the brand name Precose (since discontinued). This class of antidiabetic therapy is not widely used due to their relatively modest impact on A1c, their requirement for thrice-daily dosing, and the potential for significant gastrointestinal adverse effects.
Manufacturing Process
The title compound was obtained from a cultural broth of fermenting a
microorganism of family Actinoplanaceae in particular of strain genus
Actinoplanes, according to DT-OS (German Published Specification) No.
2,347,782.
50 g of cation exchange resin (Lewatit S 1040W) in the H+ form and 30 g an
anion exchanger (Lewatit M 600) in OH form were added to 300 ml of a
culture broth with mycelium content of 25% and an inhibitor content of 50
SIU/ml in solution. The mixture was stirred at room temperature for 50
minutes. The solution, containing a sieving nozzle with 0.1 mm slits. For testing, a sample of filtered broth was centrifuged and was examined for
saccharase inhibitor in the supernatant liquor. The solution contained 2.5
SIU/ml, that is to say 5%, of starting activity. This procedure improved the
yield of the aminosugar from 20% in the procedure known from DT-OS
(German Published Specification) No. 2,347,782, to 50-60%. It has now found
that cation exchangers based on styrene, divinylbenzene and
methoxymethylmethacrylamide, oxyethyl(meth)acrylic acid amide (ester),
dimethylaminoethyl(meth)acrylic acid amide (ester) or oxypropyl(meth)acrylic
acid amide(ester) are preferred in industrial separation procedure of acarbose.
This exchanger gave a very sharp separation of the individual homologues and
impurities. And it has now found the new biosynthesis of acarbose by using of
gen engineering methods.
brand name
Precose (Bayer);Glucobay.
Therapeutic Function
Antidiabetic
World Health Organization (WHO)
Acarbose, ana-glucosidase inhibitor, is used as an adjunctive therapy in the control of postprandial hyperglycaemia in non-insulin-dependent diabetes mellitus. Use of two or more hypoglycaemic drugs is not recommended.
General Description
Acarbose is a naturally occurring oligosaccharide,which is obtained from the microorganism Actinoplanes utahensis. It is a white to off-white powder that is soluble in water and has a pKa of 5.1. As one mightexpect, its affinity for α-glucosidase is based on it being apolysaccharide that the enzyme attempts to hydrolyze. Thisallows acarbose to act as a competitive inhibitor, which inturn reduces the intestinal absorption of starch, dextrin, anddissacharides.
It is sold as 25-, 50-, and 100-mg tablets (Precose,generics) dosed with the first bite of each meal, up to t.i.d. Acarbose potently inhibits the glucoamylase activity of MGAM α-glucosidases and the sucrase activity of α-glucosidases, where as isomaltase activity is at most moderately inhibited at concentrations in the range of those in theintestinal lumen upon oral dosing, and trehalase and lactaseare not significantly inhibited. Some inhibition of pancreaticα-amylases may also contribute to the clinical effects.
https://www.sciencedirect.com
Biochem/physiol Actions
Modified tetrasaccharide that acts as a reversible α -glucosidase inhibitor.
Pharmacokinetics
Acarbose is a complex oligosaccharide that competitively inhibits the ability of brush-border alpha-glucosidase enzymes to break down ingested carbohydrates into absorbable monosaccharides, reducing carbohydrate absorption and subsequent postprandial insulin levels. Acarbose requires the co-administration of carbohydrates in order to exert its therapeutic effect, and as such should be taken with the first bite of a meal three times daily.
Given its mechanism of action, acarbose in isolation poses little risk of contributing to hypoglycemia - this risk is more pronounced, however, when acarbose is used in conjunction with other antidiabetic therapies (e.g. sulfonylureas, insulin). Patients maintained on acarbose in addition to other antidiabetic agents should be aware of the symptoms and risks of hypoglycemia and how to treat hypoglycemic episodes. There have been rare post-marketing reports of the development of pneumatosis cystoides intestinalis following treatment with alpha-glucosidase inhibitors - patients experiencing significant diarrhea/constipation, mucus discharge, and/or rectal bleeding should be investigated and, if pneumatosis cystoides intestinalis is suspected, should discontinue therapy.
Clinical Use
Based on the structure of acarbose, it should come as nosurprise that little intact acarbose reaches the systemic circulation;instead, acarbose is extensively biotransformed by the actionof microbes and digestive enzymes in the gut. Only about35% of the radioactivity in a dose of 14C-labeled acarbose administeredorally to men was excreted in the urine, appearingas several metabolites, some of which are phase II conversionproducts of 4-methylpyrogallol (O-methyl, O-sulfate, orO-glucuronide conjugates); the methylpyrogallol fragmentarises from the terminal valienamine pseudosugar. That thesebiotransformation products are mostly formed in the gut isshown by the fact that nearly 90% of an intravenously administereddose of acarbose is excreted intact in urine.
Safety Profile
Low toxicity by ingestion,subcutaneous, and intravenous routes. Human systemiceffects: liver function impaired. When heated todecomposition it emits toxic vapors of NOx.
Veterinary Drugs and Treatments
May be useful for mild reductions in blood glucose concentrations (250 – 350 mg/dl range) in dogs and cats with non-insulin-dependent diabetes mellitus and as adjunctive treatment of insulin dependent diabetes mellitus.
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: hypoglycaemic effect possibly
enhanced and increased gastrointestinal side effects
with neomycin.
Lipid lowering agents: hypoglycaemic effect possibly
enhanced by colestyramine.
Metabolism
Oral bioavailability is 1-2%. After oral administration of the 14C-labelled substance, on average, 35% of the total radioactivity was excreted by the kidneys within 96 hours. The proportion of drug excreted in the urine was 1.7% of the administered dose. 50% of the activity was eliminated within 96 hours in the faeces.
Metabolism
Acarbose is extensively metabolized within the gastrointestinal tract, primarily by intestinal bacteria and to a lesser extent by digestive enzymes, into at least 13 identified metabolites. Approximately 1/3 of these metabolites are absorbed into the circulation where they are subsequently renally excreted. The major metabolites appear to be methyl, sulfate, and glucuronide conjugates of 4-methylpyrogallol.
Only one metabolite - resulting from the cleavage of a glucose molecule from acarbose - has been identified as having alpha-glucosidase inhibitory activity.
storage
Desiccate at RT
Properties of Acarbose
| Melting point: | 165-170°C |
| Boiling point: | 675.05°C (rough estimate) |
| alpha | D18 +165° (c = 0.4 in water) |
| Density | 1.4278 (rough estimate) |
| refractive index | 1.6000 (estimate) |
| storage temp. | 2-8°C |
| solubility | Very soluble in water, soluble in methanol, practically insoluble in methylene chloride. |
| form | neat |
| pka | 12.39±0.20(Predicted) |
| appearance | white to off-white powder |
| form | Solid |
| color | White to Off-White |
| Water Solubility | Soluble in water. |
| Merck | 14,18 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 56180-94-0 |
Safety information for Acarbose
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Acarbose
| InChIKey | XUFXOAAUWZOOIT-JMPDRRIHSA-N |
Acarbose manufacturer
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran



You may like
-
 Acarbose, ≥95% CAS 56180-94-0View Details
Acarbose, ≥95% CAS 56180-94-0View Details
56180-94-0 -
 Acarbose extrapure CAS 56180-94-0View Details
Acarbose extrapure CAS 56180-94-0View Details
56180-94-0 -
 Acarbose CAS 56180-94-0View Details
Acarbose CAS 56180-94-0View Details
56180-94-0 -
 Acarbose CAS 56180-94-0View Details
Acarbose CAS 56180-94-0View Details
56180-94-0 -
 Acarbose Hydrate >98% CAS 56180-94-0View Details
Acarbose Hydrate >98% CAS 56180-94-0View Details
56180-94-0 -
 56180-94-0 ACARBOSE 20% DC GRANULES 98%View Details
56180-94-0 ACARBOSE 20% DC GRANULES 98%View Details
56180-94-0 -
 56180-94-0 98%View Details
56180-94-0 98%View Details
56180-94-0 -
 609-15-4View Details
609-15-4View Details
609-15-4
