Acacetin
Synonym(s):4′-Methylapigenin;5,7-Dihydroxy-4′-methoxyflavone;Apigenin 4′-methyl ether;Buddleoflavonol;Linarigenin
- CAS NO.:480-44-4
- Empirical Formula: C16H12O5
- Molecular Weight: 284.26
- MDL number: MFCD00016936
- EINECS: 207-552-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-10 11:33:04
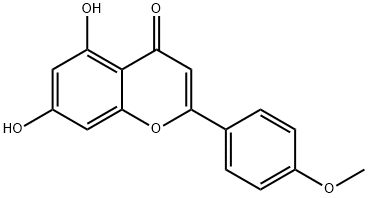
What is Acacetin?
Description
Acacetin is an O-methylated flavone found in various plants. It is reported to demonstrate spasmolytic, antinociceptive, anti-inflammatory, and antioxidant activity in various research models.
Chemical properties
Yellow Solid
The Uses of Acacetin
VEGF expression inhibitor and tumor angiogenesis. A flavonoid with antiaggregatory activity in human blood.
What are the applications of Application
Acacetin is a flavonoid with anti-peroxidative and anti-inflammatory properties
Definition
ChEBI: A monomethoxyflavone that is the 4'-methyl ether derivative of apigenin.
Synthesis Reference(s)
Tetrahedron Letters, 31, p. 6497, 1990 DOI: 10.1016/S0040-4039(00)97100-4
General Description
Acacetin belongs to the category of naturally occurring plant pigments called flavonoids found in vascular plants. It was first reported to be extracted from the leaves of Robinia pseuducacia. It has been used as an active component of traditional Chinese medicine Xuelianhua.
Biochem/physiol Actions
Acacetin has been noted to exhibit anti-peroxidative, anti-inflammatory, and anti-plasmodial properties. It has been posited to prevent the proliferation of Hep G2 cells, thereby causing cell apoptosis and subsequent anti-cancer action. It has antiarrhythmic properties and can be used in the treatment of Atrial fibrillation.
Enzyme inhibitor
This naturally occurring flavone (FW = 286.27 g/mol; CAS 480-44-4), also known as 5,7-dihydroxy-4’-methoxyflavone and 7-O-methylapigenin, and systematically named as 5,7-dihydroxy-2-(4-methoxyphenyl)-chromen-4- one, from the black locust Robinia pseudoacacia is the aglycon of linarin and acaciin and is biosynthesized by apigenin 4'-O-methyltransferase from S-adenosyl-methionine and 5,7,4’-trihydroxyflavone (apigenin), yielding Sadenosylhomocysteine and acacetin. The chemical synthesis of acacetin was accomplished by Robert Robinson, who was awarded the 1947 Nobel Prize in Chemistry for his work on alkaloids and organic synthesis. Target(s): glutathione S-transferase; xanthine oxidase, Ki = 0.11 μM; CYP1A; CYP1B1; glutathione-disulfide reductase; DNA topoisomerase I; [myosin light-chain] kinase; and protein-tyrosine kinase, or non-specific protein-tyrosine kinase.
Properties of Acacetin
| Melting point: | 260-265 °C(lit.) |
| Boiling point: | 346.76°C (rough estimate) |
| Density | 1.2160 (rough estimate) |
| refractive index | 1.6200 (estimate) |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Methanol (Very Slightly, Heated) |
| form | Solid |
| pka | 6.51±0.40(Predicted) |
| color | Light Yellow to Green-Yellow to Dark Yellow |
| λmax | 335nm(EtOH)(lit.) |
| Merck | 14,13 |
| BRN | 277879 |
| CAS DataBase Reference | 480-44-4(CAS DataBase Reference) |
Safety information for Acacetin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Acacetin
| InChIKey | DANYIYRPLHHOCZ-UHFFFAOYSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
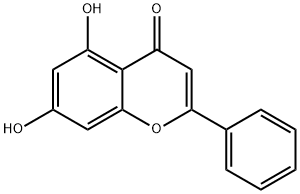
You may like
-
 480-44-4 4'-methoxy-5,7-dihydroxy flavone 98%View Details
480-44-4 4'-methoxy-5,7-dihydroxy flavone 98%View Details
480-44-4 -
 Acacetin CAS 480-44-4View Details
Acacetin CAS 480-44-4View Details
480-44-4 -
 Acacetin CAS 480-44-4View Details
Acacetin CAS 480-44-4View Details
480-44-4 -
 Acacetin CAS 480-44-4View Details
Acacetin CAS 480-44-4View Details
480-44-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1