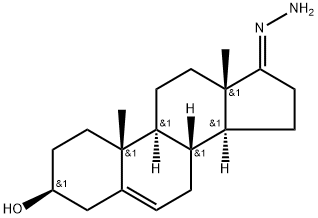
Abiraterone
- CAS NO.:154229-19-3
- Empirical Formula: C24H31NO
- Molecular Weight: 349.52
- MDL number: MFCD00924100
- EINECS: 810-941-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 08:49:36
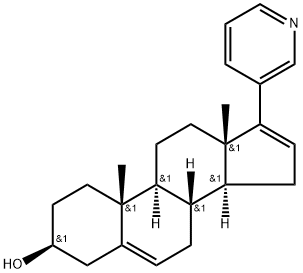
What is Abiraterone?
Absorption
Geometric mean (± SD) Cmax was 73 (± 44) ng/mL and AUC0-∞ was 373 (± 249) ng x hr/mL following a single dose of 500 mg abiraterone acetate in overnight-fasted healthy subjects. Dose proportionality was observed in single doses of abiraterone acetate ranging from 125 mg to 625 mg. A group of patients with mCRPC received a daily dose of 1,000 mg: at steady-state, the mean (± SD) Cmax was 226 (± 178) ng/mL and AUC was 993 (± 639) ng x hr/mL.
Following oral administration of abiraterone acetate to patients with metastatic castration-resistant prostate cancer, the median Tmax was two hours. In vivo, abiraterone acetate is converted to abiraterone. In clinical studies of other abiraterone acetate formulations, abiraterone acetate plasma concentrations were below detectable levels (< 0.2 ng/mL) in > 99% of the analyzed samples.
Systemic exposure to abiraterone is increased when abiraterone acetate is administered with food. Abiraterone Cmax was approximately 6.5-fold higher, and AUC0-∞ was 4.4-fold higher when a single dose of abiraterone acetate 500 mg was administered with a high-fat meal (56-60% fat, 900-1,000 calories) compared to overnight fasting in healthy subjects. Given the normal variation in the content and composition of meals, taking abiraterone with meals has the potential to result in increased and highly variable exposures.
Toxicity
The oral LD50 is > 400 mg/kg in rats and 800 mg/kg in mice.
The human experience of overdose with abiraterone is limited. Toxicity is related to the blockade of CYP17 activity. Blockade results in the accumulation of upstream mineralocorticoids like 11-deoxycorticosterone, leading to secondary hyperaldosteronism. Signs of hyperaldosteronism include fluid retention and hypokalemia. As there is no specific antidote for abiraterone overdose, overdosage should be managed with general supportive measures, including monitoring and assessment of cardiac and liver function.
Chemical properties
White Solid
Originator
Abiraterone,Cougar
The Uses of Abiraterone
Abiraterone, a steroidal cytochrome P 450 17α-hydroxylase-17,20-lyase inhibitor (CYP17), is currently undergoing phase II clinical trials as a potential drug for the treatment of androgen-dependent pr ostate cancer.
The Uses of Abiraterone
Prasteronyl Abiraterone is a dimer impurity of Abiraterone (A108490), a steroidal cytochrome P 450 17α-hydroxylase-17,20-lyase inhibitor (CYP17), is currently undergoing phase II clinical trials as a potential drug for the treatment of androgen-dependent prostate cancer.
Indications
Abiraterone is indicated for the treatment of metastatic castration-resistant prostate cancer (mCRPC) in combination with methylprednisolone or prednisone.
In Europe and Canada, it is also used in patients with mCRPC who are asymptomatic or mildly symptomatic after the failure of androgen deprivation therapy for whom chemotherapy is not yet clinically indicated. In Europe, it is used in patients whose disease has progressed on or after a docetaxel-based chemotherapy regimen. In Canada, it is used in patients who have received prior chemotherapy containing docetaxel after the failure of androgen deprivation therapy.
Abiraterone is indicated in combination with prednisone for the treatment of metastatic high-risk castration-sensitive prostate cancer (CSPC). In Europe and Canada, it may also be used in combination with prednisolone and androgen deprivation therapy in newly diagnosed patients.
In Canada and the US, abiraterone is also available in a combination product with niraparib, which is indicated with prednisone for the treatment of adults with deleterious or suspected deleterious BRCA-mutated (BRCAm) mCRPC. In Canada, this combination product is also used with prednisolone and is reserved for patients who are asymptomatic or mildly symptomatic, and in whom chemotherapy is not clinically indicated.
Background
Abiraterone is a potent, irreversible, and selective inhibitor of 17 αhydroxylase/C17,20-lyase (CYP17), an enzyme expressed in testicular, adrenal, and prostatic tumour tissues, to regulate androgen biosynthesis. Abiraterone was first approved by the FDA and EMA on April, July, and September 2011, respectively. It is used to treat metastatic castration-resistant prostate cancer and hormone-sensitive high-risk metastatic prostate cancer.
As abiraterone has poor oral bioavailability and is susceptible to hydrolysis by esterases, abiraterone acetate was developed as an orally bioavailable prodrug with enhanced stability and absorption.
Definition
ChEBI: A 3beta-sterol that is androsta-5,16-dien-3beta-ol substituted at position 17 by a 3-pyridyl group. Administered as the O-acetate, it is used for treatment of metastatic castrate-resistant prostate cance .
Manufacturing Process
Diethyl(3-pyridyl)borane (3.38 g, 23 mmol) from Aldrich Chemical Co. Ltd.
was added to a stirred solution of 3β-acetoxyandrosta-5,16-dien-17-yl
trifluoromethanesulphonate (6.94 g, 15 mmol) in THF (75 ml) containing
bis(triphenylphosphine)palladium(II) chloride (0.105 g, 0.15 mmol). An
aqueous solution of sodium carbonate (2 M, 30 ml) was then added and the
mixture heated, with stirring, by an oil bath at 80°C for 1 h, and allowed to
cool. The mixture was partitioned between diethyl ether and water, the ether
phase was dried (Na2CO3), filtered through a short plug of silica, and
concentrated. Chromatography, on elution with light petroleum-diethyl ether
(2:1), afforded the 3β-acetoxy-17-(3-pyridyl)androsta-5,16-diene (4.95 g,
84%) which crystallised from hexane, m.p. 144-145°C.
To a solution of 3β-acetoxy-17-(3-pyridyl)androsta-5,16-diene (4.90 g, 12.5
mmol) in methanol (50 ml) was added an aqueous solution of sodium
hydroxide (10% w/v, 10 ml) and the mixture heated, with stirring, on an oil
bath at 80°C for 5 min, then allowed to cool. The mixture was poured into
water, neutralised with hydrochloric acid (1 M), rebasified with saturated
sodium bicarbonate solution, and extracted with hot toluene. The toluene
extracts were combined, dried (Na2CO3), and concentrated. Chromatography,
on elution with toluene-diethyl ether (2:1) afforded the 17-(3-
pyridyl)androsta-5,16-dien-3β-ol (3.45 g, 79%) which crystallised from
toluene, m.p. 228-229°C.
Therapeutic Function
Antiandrogen
Biological Activity
abiraterone (17-(3-pyridyl)androsta-5,16-dien-3β-ol) is a potent small-molecule inhibitor of cyp17 complex (17 alpha-monooxygenase) which is a member of the cytochrome p450 family consisting of 17 alpha-hydroxylase and c17,20-lyase and catalyzing the 17 alpha-hydroxylation of intermediates of steroid biosynthesis involved in testerone sysnthesis. abiraterone inhibits cyp17 complex by binding to the haem iron of cyp17a1, which forms a 60o angle above the haem plane and packs against the central i helix with 3β-oh interacting with aspargine 202 in the f helix. abiraterone is currently being investigated to treat late stage prostate cancer, in which it consistently suppresses testosterone levels, leads to significant reduction in psa level, and prolong life in patients with castration-resistant prostate cancer (crpc).natasha m. devore and emily e. scott. structures of cytochrome p450 17a1 with prostate cancer drugs abiraterone and tok-001. nature 2012; 482: 116-120farshid dayyani, gary e. gallick, christopher j. logothetis, paul g. corn. novel therapies for metastatic castrate-resistant prostate cancer. jnci j natl cancer inst (2011); 103(22): 1665-1675
Pharmacokinetics
In vivo, abiraterone acetate is rapidly hydrolyzed to abiraterone, which mediates its pharmacological actions. Abiraterone decreases serum testosterone and other androgens. A change in serum prostate-specific antigen (PSA) levels may be observed.
Metabolism
The conversion of abiraterone acetate to abiraterone, the active metabolite, is likely to be mediated by esterases, although specific esterases have not been identified. In human plasma, the two main circulating metabolites are abiraterone sulfate, which is formed by CYP3A4 and SULT2A1, and N-oxide abiraterone sulfate, which is formed by SULT2A1. These metabolites each account for about 43% of abiraterone exposure and are pharmacologically inactive.
Properties of Abiraterone
| Melting point: | 227-228 °C(Solv: toluene (108-88-3)) |
| Boiling point: | 500.2±50.0 °C(Predicted) |
| Density | 1.14±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 14.71±0.70(Predicted) |
| color | White to Pale Yellow |
| CAS DataBase Reference | 154229-19-3 |
Safety information for Abiraterone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P233:Keep container tightly closed. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P304:IF INHALED: P312:Call a POISON CENTER or doctor/physician if you feel unwell. P321:Specific treatment (see … on this label). P330:Rinse mouth. P340:Remove victim to fresh air and keep at rest in a position comfortable for breathing. P362:Take off contaminated clothing and wash before reuse. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P403:Store in a well-ventilated place. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P501:Dispose of contents/container to..… |
Computed Descriptors for Abiraterone
Abiraterone manufacturer
Clickchem Research LLP
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 154229-19-3 98%View Details
154229-19-3 98%View Details
154229-19-3 -
 Abiraterone 98%View Details
Abiraterone 98%View Details -
 Abiraterone. 154229- 19-3 98%View Details
Abiraterone. 154229- 19-3 98%View Details
154229- 19-3 -
 Abiraterone 99%View Details
Abiraterone 99%View Details -
 154229-19-3 Abiraterone 98%View Details
154229-19-3 Abiraterone 98%View Details
154229-19-3 -
 Abiraterone CAS 154229-19-3View Details
Abiraterone CAS 154229-19-3View Details
154229-19-3 -
 Abiraterone 99% (HPLC) CAS 154229-19-3View Details
Abiraterone 99% (HPLC) CAS 154229-19-3View Details
154229-19-3 -
 Abiraterone 98% CAS 154229-19-3View Details
Abiraterone 98% CAS 154229-19-3View Details
154229-19-3
