Bicalutamide
Synonym(s):Bicalutamide (CDX);Casodex;Cosudex;N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-methylpropanamide
- CAS NO.:90357-06-5
- Empirical Formula: C18H14F4N2O4S
- Molecular Weight: 430.37
- MDL number: MFCD00869971
- EINECS: 200-001-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-27 15:38:00

What is Bicalutamide?
Absorption
Bicalutamide is well-absorbed following oral administration, although the absolute bioavailability is unknown.
Description
Bicalutamide was launched in the United Kingdom, its first worldwide market, for the treatment of advanced prostate cancer in combination with an LHRH analog or surgical castration. A non-steroidal, peripherally selective antiandrogen, bicalutamide inhibits the action of dihydrotestosterone and testosterone at target sites by competitive binding to the cytosolic androgen receptor. It was reportedly well tolerated with no significant cardiovascular and metabolic side effects due to the benefit of lacking any steroid activity. The efficacy of bicalutamide as a monotherapy has been demonstrated clinically. Promising response rates were also reported in treating colorectal, breast, pancreas and non-small cell lung cancers.
Chemical properties
Off-White Crystalline Solid
Originator
Zeneca (United Kingdom)
History
Bicalutamide was discovered in the 1980s by Tucker et al. at Imperial Chemical Industries (now AstraZeneca). Based on previous works on flutamide, key structural features required for a strong anti-androgenic activity include the presence of an electron-poor aromatic ring, attached to an amide moiety. Electron-withdrawing groups at the para and the meta position of the anilide ring are beneficial for the anti-androgenic activity as compared to monosubstituted derivatives.As far as the meta position is concerned, a chloro or trifluoromethyl substituent is the best choice. Nitro and cyano groups are the best substituents at the para position. Replacement of themethyl group at the tertiary carbinol center by a trifluoromethyl group resulted in compounds with agonistic activity. In contrast to flutamide, the amide moiety of bicalutamide was extended by a sulfur linker with a second aromatic portion. The sulfanyl, sulfinyl, and sulfonyl analogues showed the same activity.The sulfanyl group was found to be oxidized to the active metabolite sulfonyl, thus indicating the sulfonyl derivative as the biologically active entity. An unsubstituted phenylsulfonyl moiety at the eastern part, or corresponding derivatives with small substituents such as fluoro at the para position, seemed to be the best in terms of anti-androgenic activity.
The Uses of Bicalutamide
Bicalutamide (CDX) has been used as an androgen receptor (AR) antagonist in prostate, bladder cancer cell lines and human fetal skeletal muscle cells. It has also been used as a supplement in RPMI 1640 for culturing androgen-independent LNCaP (LNCaP-AI) cell line.
The Uses of Bicalutamide
Non-steroidal peripherally active antiandrogen. Used as an antiandrogen, antineoplastic (hormonal)
The Uses of Bicalutamide
adrenocortical suppressant, antineoplastic, steroid biosynthesis inhibitor
The Uses of Bicalutamide
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
Background
Bicalutamide is an oral non-steroidal anti-androgen for prostate cancer. It is comprised of a racemic mixture that is a 50:50 composition of the (R)-bicalutamide and (S)-bicalutamide enantionmers. Bicalutamide binds to the androgen receptor.
Indications
Bicalutamide is indicated in combination with a luteinizing hormone-releasing hormone (LHRH) agonist for the treatment of Stage D2 metastatic carcinoma of the prostate.
What are the applications of Application
Bicalutamide is an androgen receptor inhibitor useful in prostate cancer research
Definition
ChEBI: Bicalutamide is a sulfone that is an oral non-steroidal antiandrogen used in the treatment of prostate cancer and hirsutism.
Indications
Bicalutamide was the third nonsteroidal anti-androgen that was used for the treatment of prostate cancer. Flutamide, although effective in the treatment of prostate cancer, is a pure antagonist that also affects the hypothalamus pituitary axis, thus preventing the negative feedback mechanism of androgen. Consequently, the production of LH is increased, which subsequently stimulates the synthesis of testosterone, counteracting the effectiveness of the anti-androgen. Furthermore, the half-life of the active metabolite of flutamide, hydroxyflutamide, is fairly short, and a dosing scheme of 250 mg three times daily is therefore required. The main adverse effects reported for flutamide are gynecomastia, diarrhea, and reversible liver abnormalities. Nilutamide has a longer half-life than flutamide and therefore can be administered once daily. Adverse events reported include problems with light/dark adaptation and interstitial pneumonitis. The goal that ultimately led to the discovery of bicalutamide was the identification of a novel peripherally selective anti-androgen with longer half-life than flutamide and with better tolerability as compared to both, flutamide and nilutamide.
brand name
Casodex (AstraZeneca).
Therapeutic Function
Antitumor
General Description
Bicalutamide, N-4-cyano-3-(trifluoromethyl)phenyl-3-[(4-fluorophenyl)sulfonyl]-2-hydroxy-2-methyl-propanamide (Casodex), is more potent than flutamideand has a much longer half-life (5.9 days vs. 6 hoursfor hydroxyflutamide). Because of the longer half-life, bicalutamideis used for once-a-day (50 mg) treatment of advancedprostate cancer. Bicalutamide is available as aracemic mixture, but both animal and human studies withthe AR show that the R-enantiomer has higher affinity forthe AR than the S-enantiomer.
Biological Activity
Orally active non-steroidal androgen receptor antagonist (IC 50 = 190 nM). Displays peripheral selectivity and does not effect serum levels of LH and testosterone. Exhibits potent anticancer activity in vivo .
Biochem/physiol Actions
Bicalutamide (CDX) is a non-steriodal Androgen Receptor (AR) antagonist and a pure antiandrogen. It acts via balancing histone acetylation/deacetylation and recruitment of coregulators. Bicalutamide (CDX) abolishes androgen-mediated expression. For example, MMP13 upregulation in prostate cancer, PLZF (promyelocytic leukemia zinc finger protein), and GADD45γ (growth arrest and DNA damage inducible, gamma). Bicalutamide (CDX) is inhibited by non-genomic, transcription-independent stimulation of PI3K/AKT phosphorylation by androgens.
Mechanism of action
Bicalutamide is a racemate and its antiandrogenic activity resides almost exclusively in the (R)-enantiomer, which has an approximately fourfold higher affinity for the prostate AR than hydroxyflutamide does. The (S)-enantiomer has no antiandrogenic activity. (R)-Bicalutamide is slowly absorbed, but absorption is unaffected by food. It has a long plasma elimination half-life of 1 week and accumulates approximately 10 times in plasma during daily administration. (S)-Bicalutamide is much more rapidly absorbed and cleared from plasma. At steady state, the plasma levels of (R)-bicalutamide are 100 times higher than those of (S)-bicalutamide. Although mild to moderate hepatic impairment does not affect pharmacokinetics, evidence suggests slower elimination of (R)-bicalutamide in subjects with severe hepatic impairment.
Pharmacokinetics
Bicalutamide is an antineoplastic hormonal agent primarily used in the treatment of prostate cancer. Bicalutamide is a pure, nonsteroidal anti-androgen with affinity for androgen receptors (but not for progestogen, estrogen, or glucocorticoid receptors). Consequently, Bicalutamide blocks the action of androgens of adrenal and testicular origin which stimulate the growth of normal and malignant prostatic tissue. Prostate cancer is mostly androgen-dependent and can be treated with surgical or chemical castration. To date, antiandrogen monotherapy has not consistently been shown to be equivalent to castration.
Pharmacology
Bicalutamide is a competitive AR antagonist, which shows in vitro a lower affinity for the AR as compared to the synthetic androgen R1881 as well as the natural DHT. However it displays a fourfold higher affinity as compared to hydroxyflutamide as assessed by a binding assay. Bicalutamide inhibits the growth of the LNCaP/FGC prostate carcinoma cell line, in which hydroxyflutamide was not effective at all. In vivo anti-androgenic activity of bicalutamide was confirmed by dose-dependent weight reduction of the seminal vesicles and ventrical prostate gland in rats, followed by an antitumor efficacy using Dunning R3327-GH prostate carcinomas in intact and castrated rats.A full overview on all clinical trials including bicalutamide would be out of scope.
Clinical Use
Bicalutamide is a nonsteroidal pure antiandrogen given at a dosage of 150 mg once daily as monotherapy for the treatment of early (localized or locally advanced) nonmetastatic prostate cancer. It also can be used at a lower dosage in combination with a LHRH analogue or surgical castration for the treatment of advanced prostate cancer.
Side Effects
Bicalutamide was well tolerated in monotherapy as well as in combination. No dose-related increase in adverse events was reported. Adverse events were partially due to pharmacological effects of an anti-androgen, which include gynecomastia, breast tenderness, and hot flushes. Other non-pharmacological adverse events, with incidence equal or higher than 10% were, for example, constipation, nausea, diarrhea, asthenia, pain, and infection. The frequency of non-pharmacological adverse events was in the same range as reported for comparator in clinical trials. In contrast to flutamide, the incidence of diarrhea and liver abnormalities was much lower for bicalutamide. As compared with castration, monotherapy with bicalutamide allowed patients to maintain libido and have better physical capacity, thus resulting in better quality of life.Based on the results of the clinical trials mentioned above, bicalutamide was first approved in 1995. Bicalutamide is indicated for the use in combination with an LHRH-A analogue for metastatic prostate carcinoma (50mg).
Drug interactions
Potentially hazardous interactions with other drugs
Anticoagulants: possibly enhances anticoagulant
effect of coumarins.
Lipid lowering agents: separate lomitapide and
bicalutamide administration by 12 hours.
See 'Other information'.
Metabolism
Bicalutamide undergoes stereo specific metabolism. The S (inactive) isomer is metabolized primarily by glucuronidation. The R (active) isomer also undergoes glucuronidation but is predominantly oxidized to an inactive metabolite followed by glucuronidation.
Metabolism
Bicalutamide metabolites are excreted almost equally in urine and feces, with little or no unchanged drug excreted in urine. Unmetabolized drug predominates in the plasma. Following oral administration, the racemate displays stereoselective oxidative metabolism of its (R)-enantiomer, with an elimination half-life of approximately 6 days. (R)-Bicalutamide is cleared almost exclusively by CYP3A4-mediated metabolism, but glucuronidation is the predominant metabolic route for (S)-bicalutamide.
Storage
Store at RT
Properties of Bicalutamide
| Melting point: | 191-193°C |
| Boiling point: | 650.3±55.0 °C(Predicted) |
| Density | 1.52±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: >5mg/mL |
| form | powder |
| pka | 11.49±0.29(Predicted) |
| color | White to Off-White |
| λmax | 270nm(CH3CN)(lit.) |
| Merck | 14,1200 |
| CAS DataBase Reference | 90357-06-5(CAS DataBase Reference) |
Safety information for Bicalutamide
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H351:Carcinogenicity H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P391:Collect spillage. Hazardous to the aquatic environment P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. |
Computed Descriptors for Bicalutamide
Bicalutamide manufacturer
ALS India Life Sciences Pvt. Ltd
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)thio]-2-hydroxy-2-methylpropionamide](https://img.chemicalbook.in/CAS/GIF/90356-78-8.gif)
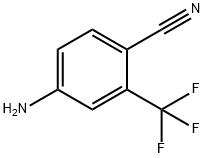
![N-[4-Cyano-3-(trifluoromethyl)phenyl]methacrylamide epoxide](https://img.chemicalbook.in/CAS/GIF/90357-51-0.gif)
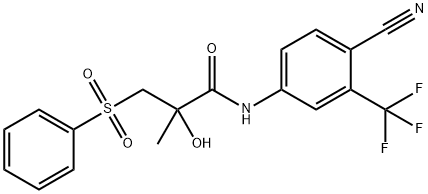

![N-[4-Cyano-3-(trifluoromethyl)phenyl]-2-methacrylamide](https://img.chemicalbook.in/CAS/GIF/90357-53-2.gif)
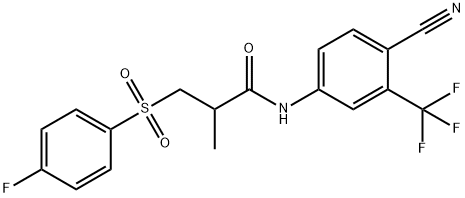

You may like
-
 90357-06-5 Bicalutamide crude 98%View Details
90357-06-5 Bicalutamide crude 98%View Details
90357-06-5 -
 Bicalutamide 98%View Details
Bicalutamide 98%View Details -
 Bicalutamide 98%View Details
Bicalutamide 98%View Details -
 Bicalutamide 99%View Details
Bicalutamide 99%View Details -
 90357-06-5 95-99%View Details
90357-06-5 95-99%View Details
90357-06-5 -
 Bicalutamide (CDX) >98% (HPLC) CAS 90357-06-5View Details
Bicalutamide (CDX) >98% (HPLC) CAS 90357-06-5View Details
90357-06-5 -
 Bicalutamide 99.59%View Details
Bicalutamide 99.59%View Details
90357-06-5 -
 Bicalutamide API 1570-45-2, CHEMLAND IND, DrumView Details
Bicalutamide API 1570-45-2, CHEMLAND IND, DrumView Details
90357-06-5
