Pictilisib
- CAS NO.:957054-30-7
- Empirical Formula: C23H27N7O3S2
- Molecular Weight: 513.64
- MDL number: MFCD11616196
- EINECS: 1312995-182-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
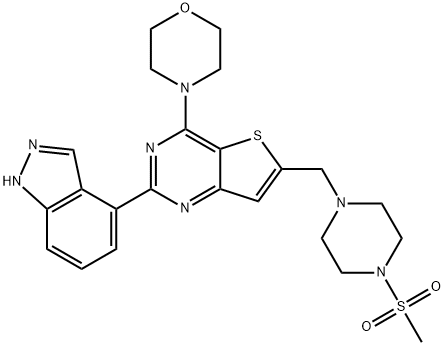
What is Pictilisib?
Description
GDC-0941/Pictilisib (957054-30-7) is a potent and selective inhibitor of class I phosphatidylinositol-3-kinases (PI3K) with significant antitumor activity – IC50’s: PI3Kα = 3nM, PI3Kβ = 33 nM, PI3Kδ = 3 nM, PI3Kγ = 75 nM.1,2?? GDC-0941 is the chemical probe of choice for the pan-inhibition of class I PI3K’s.3?Currently in clinical trials.4
The Uses of Pictilisib
GDC-0941 is a potent and selective oral inhibitor of the class I PI3K. GDC-0941 demonstrated broad spectrum of activity in breast, ovarian, lung, and prostate cancer models. Studies has also shown GDC -0941 may enhance anti-tumor activity of Docetaxel (D494420) in human breast cancer models.
The Uses of Pictilisib
GDC-0941 is a potent and selective oral inhibitor of the class I PI3K. GDC-0941 demonstrated broad spectrum of activity in breast, ovarian, lung, and prostate cancer models. Studies has also shown GDC-0941 may enhance anti-tumor activity of Docetaxel (D494420) in human breast cancer models. Potent PI3K inhibitor.
What are the applications of Application
GDC-0941 is a potent inhibitor the PI 3-kinase family
Definition
ChEBI: A sulfonamide composed of indazole, morpholine, and methylsulfonyl-substituted piperazine rings bound to a thienopyrimidine ring.
References
1) Folkes?et al.?(2008),?The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine(GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer;? J.?Med. Chem.,?51?5522 2) Raynaud?et al. (2009),?Biological properties of potent inhibitors of class I phosphatidylinositide 3-kinase: from PI-103 through PI-540, PI620 to the oral agent GDC-0941; Mol. Cancer Ther.,?8?1725 3) Knapp et al. (2013),?A public-private partnership to unlock the untargeted kinome; Nat. Chem. Biol.,?9?3 4) Sarker?et al. (2015),?First-in-human phase I study of pictilisib (GDC-0941), a potent pan-class I phosphatidylinositol-3-kinase (PI3K) inhibitor, in patients with advanced solid tumors; Clin. Cancer Res.,?21?77
Properties of Pictilisib
| Melting point: | >200oC (dec.) |
| Density | 1.53±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (>25 mg/ml) |
| pka | 12.22±0.40(Predicted) |
| form | White powder solid. |
| color | White/off-white |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
Safety information for Pictilisib
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Pictilisib
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 GDC-0941 98% (HPLC) CAS 957054-30-7View Details
GDC-0941 98% (HPLC) CAS 957054-30-7View Details
957054-30-7 -
 PI 3-K Inhibitor XXI, GDC-0941 CAS 957054-30-7View Details
PI 3-K Inhibitor XXI, GDC-0941 CAS 957054-30-7View Details
957054-30-7 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
