PLERIXAFOR
- CAS NO.:110078-46-1
- Empirical Formula: C28H54N8
- Molecular Weight: 502.78
- MDL number: MFCD05662218
- EINECS: 1592732-453-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
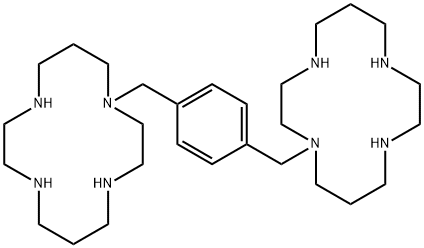
What is PLERIXAFOR?
Absorption
Plerixafor follows a two-compartment pharmacokinetic profile with first-order absorption and exhibits linear kinetics between 0.04 mg/kg and 0.24 mg/kg. The pharmacokinetic profile of plerixafor in healthy subjects was similar to the one observed in patients with non-Hodgkin’s lymphoma (NHL) and multiple myeloma (MM) who received plerixafor in combination with granulocyte-colony stimulating factor (G-CSF). In addition, the clearance of plerixafor has a significant relationship with creatinine clearance (CLCR). The population pharmacokinetic analysis showed that, with increasing body weight, a mg/kg-based dosage leads to a higher plerixafor exposure (AUC0-24h). However, NHL patients (<70 kg) given a fixed dose of 20 mg of plerixafor had an AUC0-10h 1.43-fold higher than the one detected in patients given 0.24 mg/kg of plerixafor. Therefore, a body weight of 83 kg was selected as an appropriate cut-off point to transition patients from fixed to weight-based dosing.
Peak concentrations are reached in approximately 30-60 minutes (tmax) following subcutaneous injection. In patients given 0.24 mg/kg of plerixafor subcutaneously after receiving 4-days of G-CSF pre-treatment, the Cmax and AUC0-24 were 887 ng/ml and 4337 ng·hr/ml, respectively.
Toxicity
There is limited data about the effects of plerixafor at doses higher than the recommended (0.24 mg/kg subcutaneously). However, the frequency of gastrointestinal disorders, vasovagal reactions, orthostatic hypotension, and/or syncope may be higher. The carcinogenicity of plerixafor has not been evaluated, and the effect of plerixafor on human fertility is unknown. According to the results from an in vitro bacterial mutation assay, an in vitro chromosomal aberration test, and an in vivo bone marrow micronucleus test in rats after subcutaneous doses up to 25 mg/kg, plerixafor is not genotoxic. In mice and rats, the LD50 of plerixafor by intravenous injection is 5 mg/kg. The LD50 of plerixafor by subcutaneous injection is 16 mg/kg in mice and >50 mg/kg in rats/.
Description
Autologous hematopoietic stem cell (HSC) transplantation, a standard
treatment for hematological malignancies, such as non-Hodgkin’s lymphoma or multiple myeloma, involves collection of HSCs from the
patient, chemo- or radiotherapy of the patient to eliminate malignant cells,
and retransplantation of the stored HSCs.
Plerixafor is a potent antagonist of the CXCR4 chemokine receptor and a first-in-class drug for stem cell mobilization. CXCR4
is specific for stromal-derived-factor-1 (SDF-1), a molecule endowed with
potent chemotactic activity forlymphocytes. Because the interaction between
SDF-1 and CXCR4 plays an important role in holding HSCs in the bone
marrow, drugs that block the activity of CXCR4 receptor are capable of
mobilizing HSCs into the bloodstream. In vitro, plerixafor potently blocks
SDF-1 binding of CXCR4 and inhibits SDF-1-induced calcium flux
(IC50 = 0.01-0.13 mg/mL) and chemotaxis (IC50 = 0.13 mg/mL) in several
different cell types.
Plerixafor, in combination with G-CSF, is specifically
indicated to mobilize HSCs to the peripheral blood for collection and subsequent autologous transplantation in patients with non-Hodgkin’s lymphoma and multiple myeloma.
Chemical properties
White Solid
Originator
AnorMED (Canada)
The Uses of PLERIXAFOR
Plerixafor is a chemokine receptor antagonist for CXCR4 and CXCL12-mediated chemotaxis with IC50 of 44 nM and 5.7 nM, respectively
The Uses of PLERIXAFOR
Plerixafor is a hematopoietic stem cell (HSC) mobilizer that inhibits the CXCR4 chemokine receptor and blocks binding of its ligand, stromal cell-derived factor-1-α (SDF-1-α). This agent was approved on Dec. 15, 2008, as treatment in combination with granulocyte-colony stimulating factor (G-CSF) to mobilize HSCs to the peripheral blood for collection and subsequent autologous transplantation in patients with non-Hodgkin''s lymphoma (NHL) and multiple myeloma (MM). Selective CXCR4 antagonist.
Indications
Plerixafor is indicated in combination with granulocyte-colony stimulating factor (G-CSF) to mobilize hematopoietic stem cells (HSCs) to the peripheral blood for collection and subsequent autologous transplantation in patients with non-Hodgkin’s lymphoma or multiple myeloma.
Background
Plerixafor is a small-molecule inhibitor of C-X-C chemokine receptor type 4 (CXCR4) that acts as a hematopoietic stem cell mobilizer. It is used to stimulate the release of stem cells from the bone marrow into the blood in patients with non-Hodgkin's lymphoma (NHL) and multiple myeloma to stimulate their immune system. These stem cells are then collected and used in autologous stem cell transplantation to replace blood-forming cells destroyed by chemotherapy.
As an inhibitor of CXCR4, plerixafor blocks the binding of its ligand, stromal cell-derived factor-1-alpha (SDF-1α). Since CXCR4 and SDF-1α are involved in the trafficking and homing of CD34+ cells to the marrow compartment, blocking this interaction leads to an increase in CD34+ cell circulating levels. Compared to placebo with G-CSF, the plerixafor and G-CSF mobilization regimen has a higher probability of achieving the optimal CD34+ cell target for tandem transplantation in fewer apheresis procedures.
Plerixafor has orphan drug status in the United States and European Union and was approved by the US Food and Drug Administration on December 15, 2008.
Definition
ChEBI: Plerixafor is an azamacrocycle consisting of two cyclam rings connected by a 1,4-phenylenebis(methylene) linker. It is a CXCR4 chemokine receptor antagonist and a hematopoietic stem cell mobilizer. It is used in combination with grulocyte-colony stimulating factor (G-CSF) to mobilize hematopoietic stem cells to the perpheral blood for collection and subsequent autologous transplantation in patients with non-Hodgkin's lymphoma and multiple myeloma. It has a role as an immunological adjuvant, an antineoplastic agent, an anti-HIV agent and a C-X-C chemokine receptor type 4 antagonist. It is an azamacrocycle, a crown amine, a secondary amino compound, an azacycloalkane, a tertiary amino compound and a member of benzenes. It is functionally related to a 1,4,8,11-tetraazacyclotetradecane.
brand name
Mozobil
Pharmacokinetics
Plerixafor is a bicyclam derivative that antagonizes C-X-C chemokine receptor type 4 (CXCR4) by binding to three acidic residues in the ligand-binding pocket: Asp171, Asp262, and Glu288. Blood levels of CD34+ cells peaked between 6 and 9 hours after the administration of 0.24 mg/kg plerixafor in healthy subjects. In combination with a granulocyte-colony stimulating factor (G-CSF), circulating CD34+ cells in the peripheral blood peaked between 10 and 14 hours. The use of plerixafor is not associated with QT/QTc prolongation at single doses up to 0.40 mg/kg.
Serious hypersensitivity reactions, such as anaphylactic-type reactions, have occurred in patients receiving plerixafor. The use of plerixafor may also cause tumor cell mobilization in leukemia patients, splenic enlargement and rupture, embryo-fetal toxicity, and hematologic effects, such as leukocytosis and thrombocytopenia. When used in combination with G-CSF for hematopoietic stem cell mobilization? plerixafor may lead to the release of tumor cells from the marrow and their subsequent collection in the leukapheresis product.
Clinical Use
Chemokine receptor antagonist:
To enhance mobilisation of haematopoietic stem
cells to the peripheral blood for collection and
subsequent autologous transplantation in patients
with lymphoma and multiple myeloma whose cells
mobilise poorly
Side Effects
The most common adverse reactions (occurring in more than 10% of patients) observed during plerixafor use include diarrhea, nausea, fatigue, injection-site reactions, headache, arthralgia, dizziness, and vomiting.
Synthesis
Plerixafor can be synthesized by several related procedures starting from tetraazacyclotetradecane, the macrocyclic tetraamine cyclam. The general synthetic strategy entails the masking of three of the four ring nitrogens, followed by alkylation with para-xylylene dibromide, and subsequent removal of the masking groups. In one approach, tetraazacyclotetradecane is protected as the phosphorotriamide derivative by reaction with tris (dimethylamino)phosphine followed by oxidation with carbon tetrachloride and sodium hydroxide. After condensation with xylylene dibromide, the dimeric bis-phosphoramide intermediate is hydrolyzed to plerixafor by treatment with dilute hydrochloric acid solution.
Metabolism
Plerixafor is not metabolized by the liver and is not a metabolism-dependent inhibitor of major cytochrome P450 enzymes, including 1A2, 2C9, 2C19, 2D6 and 3A4. In addition, it does not induce cytochrome P450 1A2, 2B6, or 3A4 enzymes. Plerixafor is metabolically stable, and in vivo studies in rats and dogs showed that the non-parent radiolabelled components in plasma and urine were Cu2+ complexes with plerixafor. This is consistent with the presence of two cyclam rings in plerixafor, which may act as potential chelating sites.
Metabolism
Not metabolised.
About 70% of a dose is eliminated in the urine within 24
hours.
References
[1]zabel ba, wang y, lewén s, berahovich rd, penfold me, zhang p, powers j, summers bc, miao z, zhao b, jalili a, janowska-wieczorek a, jaen jc, schall tj. elucidation of cxcr7-mediated signaling events and inhibition of cxcr4-mediated tumor cell transendothelial migration by cxcr7 ligands. j immunol. 2009 sep 1;183(5):3204-11.
[2].li j, oupicky d. effect of biodegradability on cxcr4 antagonism, transfection efficacy and antimetastatic activity of polymeric plerixafor.biomaterials. 2014 jul;35(21):5572-9.
[3]. broxmeyer he. chemokines in hematopoiesis. curr opin hematol. 2008 jan;15(1):49-58.
[4]. devi s, wang y, chew wk, lima r, a-gonzález n, mattar cn, chong sz, schlitzer a, bakocevic n, chew s, keeble jl, goh cc, li jl, evrard m, malleret b, larbi a, renia l, haniffa m, tan sm, chan jk, balabanian k, nagasawa t, bachelerie f, hidalgo a, ginhoux f, kubes p, ng lg. neutrophil mobilization via plerixafor-mediated cxcr4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. j exp med. 2013 oct 21;210(11):2321-36.
[5]. mcdermott dh, liu q, velez d, lopez l, anaya-o'brien s, ulrick j, kwatemaa n, starling j, fleisher ta, priel da, merideth ma, giuntoli rl, evbuomwan mo, littel p, marquesen mm, hilligoss d, decastro r, grimes gj, hwang st, pittaluga s, calvo kr, stratton p, cowen ew, kuhns db, malech hl, murphy pm. a phase 1
Properties of PLERIXAFOR
| Melting point: | 122-125°C |
| Boiling point: | 657.5±55.0 °C(Predicted) |
| Density | 0.962 |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | Methanol (Slightly), Water (Slightly) |
| form | Solid |
| pka | 10.60±0.20(Predicted) |
| color | White to Pale Beige |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 110078-46-1 |
Safety information for PLERIXAFOR
Computed Descriptors for PLERIXAFOR
PLERIXAFOR manufacturer
Aspen Biopharma Labs Pvt Ltd
Related products of tetrahydrofuran


You may like
-
 110078-46-1 Plerixafor 98%View Details
110078-46-1 Plerixafor 98%View Details
110078-46-1 -
 110078-46-1 98%View Details
110078-46-1 98%View Details
110078-46-1 -
 Plerixafor 95% CAS 110078-46-1View Details
Plerixafor 95% CAS 110078-46-1View Details
110078-46-1 -
 Plerixafor 98%View Details
Plerixafor 98%View Details
110078-46-1 -
 110078-46-1 98%View Details
110078-46-1 98%View Details
110078-46-1 -
 110078-46-1 98%View Details
110078-46-1 98%View Details
110078-46-1 -
 Plerixafor CAS 110078-46-1View Details
Plerixafor CAS 110078-46-1View Details
110078-46-1 -
 Plerixafor CAS 110078-46-1View Details
Plerixafor CAS 110078-46-1View Details
110078-46-1