7-hydroxy-4-Methyl-2-oxo-2H-1-Benzopyran-8-carboxaldehyde
Synonym(s):7-Hydroxy-4-methyl-2-oxo-2H-1-benzopyran-8-carboxaldehyde;8-Formyl-7-hydroxy-4-methylcoumarin, 7-Hydroxy-4-methyl-2-oxo-2H-chromene-8-carbaldehyde, ER-to-Nucleus Signaling 1 Inhibitor III, Inositol-Reguiring Protein 1 Inhibitor III;IRE1 Inhibitor III, 4μ8C - CAS 14003-96-4 - Calbiochem
- CAS NO.:14003-96-4
- Empirical Formula: C11H8O4
- Molecular Weight: 204.18
- MDL number: MFCD12027255
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
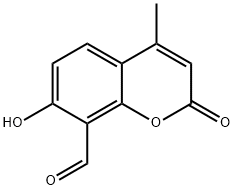
What is 7-hydroxy-4-Methyl-2-oxo-2H-1-Benzopyran-8-carboxaldehyde?
Description
4μ8C (14003-96-4) is a selective inhibitor of IRE1α ribonuclease (RNase) activity (IC50?= 60 nM). Covalently binds to lysine 907 in the IRE1 endonuclease domain, blocking substrate access to the active site of IRE1α and inactivating both XBP1 splicing and IRE1α-mediated mRNA degradation but not IRE1 kinase activity.1?Inhibits IRE1α in response to hypoxia or other ER stress-inducing agents but has no effect on proliferation or clonogenic survival of hypoxic cells.2?Blocks production of IL-4, IL-5 and IL-13 production in T cells.3?4μ8C prevents the splicing of the XBP1 mRNA in response to ER stress caused by mutant proinsulin production in pancreatic β-cells.4
The Uses of 7-hydroxy-4-Methyl-2-oxo-2H-1-Benzopyran-8-carboxaldehyde
8-Formyl-4-methylumbelliferone is an inhibitor that blocks substrate access to the active site of IRE1 and selectively inactivates both Xbp1 splicing and IRE1-mediated mRNA degradation.
General Description
A cell-permeable coumarin o-hydroxyaldehyde compound that inhibits IRE1 RNase activity in a time- and dose-dependent manner (IC50 = 550, 230, 180, 100, and 45 nM, respectively, with 0, 2, 4, 8, 16, min drug preincubation in FRET-based RNA cleavage assays) by covalently targeting IRE1 Lys907 via Schiff base formation, effectively preventing ER stress-induced site-specific mRNA splicing as well as RIDD (Regulated IRE1-Dependent Degradation) mRNA degradation (IC50 = 6.9 and 4.1 μM, respectively, against Xbp1 splicing and Scara3 degradation) in MEF cultures following Tunicamycin (Cat. No. 654380) treatment. Also demonstrated to inhibit ER capacity expansion (Effective conc. 32 μM) and amylase secretion (IC50<2 μM) upon stress induction by Dexamethasone (Cat. No. 265005) treatment in rat AR42J tumoral acinar pancreatic cells. Structural analysis reveals that the reduced water accessibility to Lys907 in IRE1 native conformation accounts for the unusual stability of Lys907 Schiff base formation and forms the basis of selective IRE1 RNase inhibition by 4μ8C and STF083010 (Cat. No. 412510). Although 4μ8C, but not STF083010, is also shown to inhibit IRE1 autophosphorylation by Schiff base formation with IRE1 Lys599 in the absence of ADP, cellular nucleotide prevents 4μ8C from targeting IRE1 Lys599 and inhibiting IRE1 kinase activity intracellularly.
Biochem/physiol Actions
Cell permeable: yes
storage
Store at -20°C
References
1) Cross?et al. (2012),?The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule; Proc. Natl. Acad. Sci. USA,?109?E869 2) Cojocari?et al. (2013),?New small molecule inhibitors of UPR activation demonstrate that PERK, but not IRE1α signaling is essential for promoting adaptation and survival to hypoxia;? Radiother. Oncol.,?108?541 3) Kemp?et al. (2013),?The serine-threonine kinase inositol-requiring enzyme 1α (IRE1α) promotes IL-4 production in T helper cells; J. Biol. Chem.,?288?33272 4) Zhang?et al. (2014),?IRE1 inhibition perturbs the unfolded protein response in a pancreatic β-cell line expressing mutant proinsulin, but does not sensitize the cells to apoptosis;? BMC Cell Biol.,?15?29
Properties of 7-hydroxy-4-Methyl-2-oxo-2H-1-Benzopyran-8-carboxaldehyde
| Melting point: | 189-190℃ (ethanol ) |
| Boiling point: | 398.3±42.0 °C(Predicted) |
| Density | 1.541 |
| refractive index | 1.641 |
| storage temp. | -20°C |
| solubility | DMSO: soluble5mg/mL, clear (warmed) |
| pka | 6.22±0.20(Predicted) |
| form | Yellow powder |
| color | white to beige |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 1 month. |
| CAS DataBase Reference | 14003-96-4 |
Safety information for 7-hydroxy-4-Methyl-2-oxo-2H-1-Benzopyran-8-carboxaldehyde
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H350:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P285:In case of inadequate ventilation wear respiratory protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. P342+P311:IF experiencing respiratory symptoms: call a POISON CENTER or doctor/physician. P403+P233:Store in a well-ventilated place. Keep container tightly closed. |
Computed Descriptors for 7-hydroxy-4-Methyl-2-oxo-2H-1-Benzopyran-8-carboxaldehyde
| InChIKey | RTHHSXOVIJWFQP-UHFFFAOYSA-N |
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![7Methyl-5-(1-{[3-(trifluoroMethyl)phenyl]acetyl}-2,3-dihydro1Hindol-5-yl)7Hpyrrolo[2,3d]pyriMidin-4-aMine](https://img.chemicalbook.in/CAS/GIF/1337531-36-8.gif)

You may like
-
 IRE1 Inhibitor III, 4μ8C CAS 14003-96-4View Details
IRE1 Inhibitor III, 4μ8C CAS 14003-96-4View Details
14003-96-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8