6-Shogaol
Synonym(s):(6)-Shogaol;[6]-Shogaol;1-(4-Hydroxy-3-methoxyphenyl)-4-decen-3-one
- CAS NO.:555-66-8
- Empirical Formula: C17H24O3
- Molecular Weight: 276.37
- MDL number: MFCD01736094
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-01 18:09:03
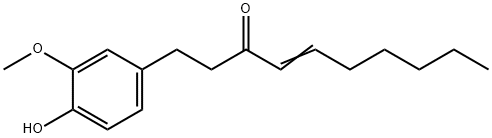
What is 6-Shogaol?
Description
Gingerol (correctly, [6]-gingerol) is the predominant phenol and most important of the pungent constituents in ginger oil. It was isolated by J. C. Thresh in 1879 from the rhizome of the ginger plant (Zingiber officinale). It and its dehydrated analogue [6]-shogaol are the primary ginger-derived bioactive compounds. Shogaol and the fragmented molecule zingerone are produced when fresh ginger is heated or cooked.?A recently synthesized azagingerol analogue?increases metabolism in mice and may reduce the risk of obesity-associated diseases.
Zingerone gives ginger a "hot" taste. It''s also an antioxidant, although it only weakly inhibits peroxidation of phospholipid liposomes in the presence of Fe(III) and ascorbate. Zingerone''s vanillin foundation and hydrocarbon tail make it a chemical relative of?eugenol?and capsaicin.
Chemical properties
White crystalline powder. B.P. higher than 300℃. Very slightly soluble in water, soluble in alcohol and oils.
The Uses of 6-Shogaol
[6]-Shogaol is an aromatic constituent of ginger and the chain-dehydroxylated analog of [6]-Gingerol. [6]-Shogaol has activity very similar to [6]-Gingerol and produced an inhibition of spontaneous motor activity, antipyretic and analgesic effects, and prolonged hexobarbital-induced sleeping time. [6]-Shogaol also has potent antitussive activity and affected the cortical EEG.
Definition
ChEBI: [6]-Shogaol is a monomethoxybenzene, a member of phenols and an enone.
What are the applications of Application
Shogaol has little or no application in perfumery, and very little interest to the flavor industry, but it is included in this work mainly for the purpose of elucidating the problems apparently existing around the description of the odorand flavor of "Zingerone".
Preparation
6-Shogaol is prepared by condensation of Vanillin with Acetone, followed by hydrogenation. The resulting ketone is then condensed with Hexaldehyde under conditions which inactivate the hydroxyl group during the reaction.
Anticancer Research
6-Shogaol is the dehydrated product of 6-gingerol, extracted from the rhizome ofginger. Treatment of HCC cell line with 6-shogaol resulted in cells with apoptoticphenotypes, which showed signs of cell and nuclear shrinkage as well as substantialchromatin condensation. De-phosphorylation of PERK and activation of theexpression of CHOP initiate caspase cascade reaction inducing apoptosis inHCC. Two-dimensional gel electrophoretic analysis of proteome revealed that in response to the treatment with 6-shogaol, a significant stimulation was observed inproteins related to the ER stress, signifying that apoptosis induced by 6-shogoal didinvolve ER stress. Cells showed marked rise in the UPR target expression, HSP70,Grp94, Grp78/Bip and the other ER chaperones on exposure to 6-shogoal in a time-dependentmanner, which elicited activation of caspase-3 and degradation of polyADP ribose polymerase (PARP). Various ER chaperone proteins improve adaptationof cancer cells to hypoxic environment and aid in developing resistance againstanticancer therapy (Zorzi and Bonvini 2011; Urra et al. 2016). Screening of specificinhibitors of Grp78 as antitumour agents (Hu et al. 2012; Liu et al. 2013; Venkatesanet al. 2015) implies that inhibition of Grp78/Bip is a very promising anticancerstrategy. HCC cells are selectively killed by 6-shogaol in the absence of anynoticeable toxic consequence on normal healthy cells and very little toxicity asstudied on SMMC7721 xenograft mice. Administration of 6-shogaol and salubrinaltogether for distinct time intervals resulted in significant increase in ER stress in thecell. It appears that 6-shogaol in combination with salubrinal has great therapeuticvalue against various malignancies including HCC (Hu et al. 2012).
Properties of 6-Shogaol
| Boiling point: | 427.5±35.0 °C(Predicted) |
| Density | 1.0448 g/cm3(Temp: 25 °C) |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| form | neat |
| pka | 10.01±0.20(Predicted) |
| form | Liquid |
| color | Colourless to Light Yellow |
| Odor | Mild, warm-herbaceous, sweet odor |
| BRN | 2056098 |
| InChI | InChI=1S/C17H24O3/c1-3-4-5-6-7-8-15(18)11-9-14-10-12-16(19)17(13-14)20-2/h7-8,10,12-13,19H,3-6,9,11H2,1-2H3 |
| CAS DataBase Reference | 555-66-8(CAS DataBase Reference) |
| NIST Chemistry Reference | 6-shogaol(555-66-8) |
Safety information for 6-Shogaol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 6-Shogaol
| InChIKey | OQWKEEOHDMUXEO-BQYQJAHWSA-N |
| SMILES | C(C1=CC=C(O)C(OC)=C1)CC(=O)C=CCCCCC |
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
![[6]-Shogaol CAS 555-66-8](https://img.chemicalbook.in//Content/image/CP5.jpg) [6]-Shogaol CAS 555-66-8View Details
[6]-Shogaol CAS 555-66-8View Details
555-66-8 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8