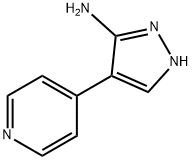
6-[4-(2-PIPERIDIN-1-YLETHOXY)PHENYL]-3-PYRIDIN-4-YLPYRAZOLO[1,5-A]PYRIMIDINE
Synonym(s):Compound C;Dorsomorphin, AMPK Inhibitor I, BMP Inhibitor I;InSolution ;6-(4-(2-Piperidin-1-yl-ethoxy)-phenyl)-3-pyridin-4-ylpyrazolo[1,5-a]pyrimidine, 2HCl, H?O, Compound C, 2HCl, H?O, AMPK Inhibitor I;6-[4-(2-Piperidin-1-yl-ethoxy)-phenyl)]-3-pyridin-4-yl-pyrrazolo[1,5-a]-pyrimidine, Dorsomorphin, BMP Inhibitor I, AMPK Inhibitor I
- CAS NO.:866405-64-3
- Empirical Formula: C24H25N5O
- Molecular Weight: 399.49
- MDL number: MFCD08705402
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
![6-[4-(2-PIPERIDIN-1-YLETHOXY)PHENYL]-3-PYRIDIN-4-YLPYRAZOLO[1,5-A]PYRIMIDINE Structural](https://img.chemicalbook.in/CAS/GIF/866405-64-3.gif)
What is 6-[4-(2-PIPERIDIN-1-YLETHOXY)PHENYL]-3-PYRIDIN-4-YLPYRAZOLO[1,5-A]PYRIMIDINE?
Description
Dorsomorphin (866405-64-3) is a potent, selective and ATP-competitive inhibitor of AMP-activated protein kinase (AMPK) (Ki = 109 nM).1 It displays no significant inhibition of ZAPK, SYK, PKCT, PKA and JAK3. It inhibits bone morphogenetic protein (BMP) type I receptors (ALK2, ALK3 and ALK6).2 Dorsomorphin has been shown to promote cardiomyogenesis in mouse embryonic stem cells (ESCs) in vitro.3 Dorsomorphin also induces autophagy in cancer cell lines via a mechanism independent of AMPK inhibition.4
The Uses of 6-[4-(2-PIPERIDIN-1-YLETHOXY)PHENYL]-3-PYRIDIN-4-YLPYRAZOLO[1,5-A]PYRIMIDINE
Dorsomorphin has been used:
- as an inhibitor of adenosine monophosphate-activated protein kinase (AMPK) to find the effects of trans-resveratrol on lipid mobilization in 3T3-L1 (a murine cell line of adipocytes) cells
- as an AMPK inhibitor, to indicate the involvement of AMPK/mTOR (mammalian target of rapamycin) pathway in LRG (liraglutide) -induced autophagy
- in the induction step, employed in in vitro differentiation of Friedreich′s ataxia (FRDA) and induced pluripotent stem cells (iPSCs) to neurospheres and neurons using ES (embryonic stem cell) media
The Uses of 6-[4-(2-PIPERIDIN-1-YLETHOXY)PHENYL]-3-PYRIDIN-4-YLPYRAZOLO[1,5-A]PYRIMIDINE
A potent inhibitor of AMPK and fatty acid biosynthesis.
What are the applications of Application
BML-275 is a Cell-permeable pyrrazolopyrimidine derivative and potent, selective, ATP-competitive inhibitor of AMPK in fatty acid biosynthesis.
Definition
ChEBI: A pyrazolopyrimidine that is pyrazolo[1,5-a]pyrimidine which is substituted at positions 3 and 6 by pyridin-4-yl and p-[2-(piperidin-1-yl)ethoxy]phenyl groups, respectively. It is a potent, selective, reversible, and ATP-competiti e inhibitor of AMPK (AMP-activated protein kinase, EC 2.7.11.31) and a selective inhibitor of bone morphogenetic protein (BMP) signaling.
General Description
A cell-permeable pyrrazolopyrimidine compound that inhibits against KDR/VEGFR2, ALK2/BMPR-I, AMPK kinase activity (IC50 = 25.1, 148, and 234.6 nM, respectively), while exhibiting much reduced or little effect toward ALK5/TGFβR-I, ZAPK, Syk, PKCθ, PKA, or JAK3. Shown to block both BMP pathway-dependent dorsoventral development (EC100 = 2.5 μM) and VEGF signaling-dependent intersomitic vessel formation (EC50 = 5 μM) in zebrafish embryo in vivo. Commonly used in combination with AMPK activators AICAR (Cat. No. 123040) and/or Metformin (Cat. No. 317240) for studying AMPK-dependent cellular events in vitro and physiological responses in animals in vivo.
Biological Activity
Potent and selective inhibitor of AMP-activated protein kinase (AMPK) (K i = 109 nM). Displays no significant activity on several structurally related kinases including ZAPK, SYK, PKC θ , PKA and JAK3. Inhibits AMPK activation induced by AICAR and metformin. Also inhibits bone morphogenic protein (BMP) type I receptors (ALK2, ALK3 and ALK6). Promotes cardiomyogenesis in mouse embryonic stem cells (ESCs) in vitro .
Biochem/physiol Actions
Target IC50: 25.1, 148, and 234.6 nM, against KDR/VEGFR2, ALK2/BMPR-I, AMPK kinase activity, respectively
References
1) Zhou et al. (2001) Role of AMP-activated protein kinase in mechanism of metformin action; J. Clin. Invest. 108 1167 2) Yu et al. (2008) Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism; Nat. Chem. Biol. 4 33 3) Hao et al. (2008) Dorsomorphin, a selective small molecule inhibitor of BMP signaling, promotes cardiomyogenesis in embryonic stem cells; PLoS ONE, 3 2904 4) Vucicevic et al. (2011) Compound C induces protective autophagy in cancer cells through AMPK inhibition-independent blockade of Akt/mTOR pathway; Autophagy 7 40
Properties of 6-[4-(2-PIPERIDIN-1-YLETHOXY)PHENYL]-3-PYRIDIN-4-YLPYRAZOLO[1,5-A]PYRIMIDINE
| Melting point: | >228°C(dec.) |
| Density | 1.25±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: >2mg/mL (warmed) |
| form | powder |
| pka | 8.53±0.10(Predicted) |
| color | white to beige |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
Safety information for 6-[4-(2-PIPERIDIN-1-YLETHOXY)PHENYL]-3-PYRIDIN-4-YLPYRAZOLO[1,5-A]PYRIMIDINE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. |
Computed Descriptors for 6-[4-(2-PIPERIDIN-1-YLETHOXY)PHENYL]-3-PYRIDIN-4-YLPYRAZOLO[1,5-A]PYRIMIDINE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![6-[4-(2-PIPERIDIN-1-YLETHOXY)PHENYL]-3-PYRIDIN-4-YLPYRAZOLO[1,5-A]PYRIMIDINE](https://img.chemicalbook.in/CAS/GIF/866405-64-3.gif)
You may like
-
 Dorsomorphin CAS 866405-64-3View Details
Dorsomorphin CAS 866405-64-3View Details
866405-64-3 -
 Dorsomorphin CAS 866405-64-3View Details
Dorsomorphin CAS 866405-64-3View Details
866405-64-3 -
 Dorsomorphin 95% CAS 866405-64-3View Details
Dorsomorphin 95% CAS 866405-64-3View Details
866405-64-3 -
 AMPK Inhibitor, Compound C CAS 866405-64-3View Details
AMPK Inhibitor, Compound C CAS 866405-64-3View Details
866405-64-3 -
 AMPK Inhibitor, Compound C•2HCl CAS 866405-64-3View Details
AMPK Inhibitor, Compound C•2HCl CAS 866405-64-3View Details
866405-64-3 -
 Dorsomorphin CAS 866405-64-3View Details
Dorsomorphin CAS 866405-64-3View Details
866405-64-3 -
 AMPK Inhibitor, Compound C CAS 866405-64-3View Details
AMPK Inhibitor, Compound C CAS 866405-64-3View Details
866405-64-3 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1