Thapsigargin
Synonym(s):;InSolution Thapsigargin - CAS 67526-95-8 - Calbiochem;Thapsigargin - CAS 67526-95-8 - Calbiochem
- CAS NO.:67526-95-8
- Empirical Formula: C34H50O12
- Molecular Weight: 650.75
- MDL number: MFCD00083511
- EINECS: 614-076-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
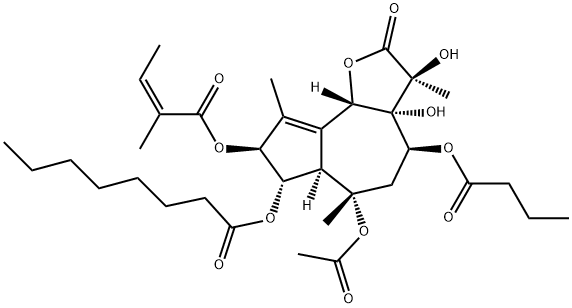
What is Thapsigargin?
Description
Thapsigargin is a naturally occurring sesquiterpene lactone isolated from the umbelliferous plant Thapsia garganica. It is a non-competitive, cell permeable inhibitor of calcium transport by SERCAs (IC50 values are cell type-dependent and range from ~2-80 nM). This tumor promoter releases Ca2+ from intracellular stores by specifically inhibiting the endoplasmic reticulum Ca2+-ATPase; it does not directly affect plasma membrane Ca2+-ATPases, Ins 1,4,5-P3 production or protein kinase C activity. This effect is a result of emptying the intracellular calcium stores, which leads to a chain of events that causes apoptosis.
Chemical properties
Thapsigargin (TG) is colourless film. It is extracted from the plant Thapsia garganica L. with methanol and purified by HPLC. TG is a tumor promoting plant sesquiterpene lactone extract with a unique biological activity as Ca2+ -ATPase inhibitors in animal cells. It is a skin irritant, a platelet activating, inflammatory and tumor promoting agent. TG is toxic and a possible carcinogen.
The Uses of Thapsigargin
THAPSIGARGIN is a widely used inhibitor of the ubiquitous sarco-endoplasmic reticulum Ca(2+)-ATPases in mammalian cells. It acts as a potent, cell-permeable, IP3-independent intracellular calcium releaser that blocks the transient increase in intracellular Ca2+ induced by angiostatin and endostatin. It induces apoptosis by disrupting intracellular free Ca2+ levels.
What are the applications of Application
Thapsigargin is a cell-permeable sesquiterpene lactone inducer of intracellular stored Ca2+ release
Definition
ChEBI: Thapsigargin is an organic heterotricyclic compound that is a hexa-oxygenated 6,7-guaianolide isolated fron the roots of Thapsia garganica L., Apiaceae. A potent skin irritant, it is used in traditional medicine as a counter-irritant. Thapsigargin inhibits Ca(2+)-transporting ATPase mediated uptake of calcium ions into sarcoplasmic reticulum and is used in experimentation examining the impacts of increasing cytosolic calcium concentrations. It has a role as an EC 3.6.3.8 (Ca(2+)-transporting ATPase) inhibitor and a calcium channel blocker. It is a sesquiterpene lactone, an organic heterotricyclic compound and a butyrate ester.
Biochem/physiol Actions
Thapsigargin is an effective inhibitor of calcium ion pumps located on sarcoplasmic reticulum (SR) and endoplasmic reticulum (ER) microsomes of skeletal, cardiac, muscle and brain tissues. The inhibition of calcium ion pumps causes intracellular increase of calcium ion levels. It was found that thapsigargin at 100 nM concentration effectively inhibited the SR Ca2+- adenosine triphosphatase (ATPase) in cardiac and skeletal muscles. Thapsigargin was also reported to be effective in blocking autophagy by interfering with the autophagosome-lysosome fusion.
storage
Store at -20°C
Mode of action
The actual mechanism of action of thapsigargin is thought actually to block calcium channels in an open conformation that results in excess calcium entry from outside the cell. Excess calcium activates DNAses and precipitates the natural death sequence of the cells.Scientists are now thinking of modifying thapsigargin in such a way thatit will kill prostate cells preferentially and will not penetrate healthy cells elsewhere in the body.At the same time, they are determining whether this drug can also induce cell death in breast cancer cells.
References
1) Treiman et al. (1998), A tool coming of age: thapsigargin as an inhibitor of sarco-endoplasmic reticulum Ca(2+)-ATPases; Trends Pharmacol. Sci., 19 131 2) Zhou et al. (2009), Autophagy-mediated insulin receptor down-regulation contributes to endoplasmic reticulum stress-induced insulin resistance; Mol. Pharmacol., 76 596
Properties of Thapsigargin
| Boiling point: | 597.77°C (rough estimate) |
| Density | 1.1521 (rough estimate) |
| RTECS | RH0325700 |
| refractive index | 1.6390 (estimate) |
| storage temp. | -20°C |
| solubility | DMSO: soluble |
| form | liquid or film |
| pka | 10.57±0.70(Predicted) |
| color | Colorless |
| Sensitive | Light Sensitive |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 1 week. |
| CAS DataBase Reference | 67526-95-8(CAS DataBase Reference) |
Safety information for Thapsigargin
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Thapsigargin
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran

You may like
-
 Thapsigargin CAS 67526-95-8View Details
Thapsigargin CAS 67526-95-8View Details
67526-95-8 -
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 131987-69-4 98+View Details
131987-69-4 98+View Details
131987-69-4 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 764-60-3 2-Hexyn-1-ol 98+View Details
764-60-3 2-Hexyn-1-ol 98+View Details
764-60-3 -
 2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
2-Propanamine, 1-chloro-, hydrochloride (9CI) 98+View Details
5968-21-8 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
