5,7-Dihydroxy-4-methylcoumarin
- CAS NO.:2107-76-8
- Empirical Formula: C10H8O4
- Molecular Weight: 192.17
- MDL number: MFCD00016966
- EINECS: 218-289-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
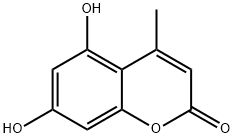
What is 5,7-Dihydroxy-4-methylcoumarin?
Chemical properties
White metallic powder
The Uses of 5,7-Dihydroxy-4-methylcoumarin
5,7-Dihydroxy-4-methylcoumarin may be used in the synthesis of pyrano[2,3-h]coumarin derivatives and 5,7-dihydroxy-8-formyl-4-methylcoumarin.
Definition
ChEBI: 5,7-Dihydroxy-4-methylcoumarin is a hydroxycoumarin.
General Description
Yellow powder. Fluoresces blue. Absorbs ultraviolet light.
Air & Water Reactions
Slightly water soluble .
Reactivity Profile
5,7-Dihydroxy-4-methylcoumarin is a phenol and lactone. Phenols do not behave as organic alcohols, as one might guess from the presence of a hydroxyl (-OH) group in their structure. Instead, they react as weak organic acids. Phenols and cresols are much weaker as acids than common carboxylic acids (phenol has Ka = 1.3 x 10^[-10]). These materials are incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat of the reaction may ignite the gas. Heat is also generated by the acid-base reaction between phenols and bases. Such heating may initiate polymerization of the organic compound. Phenols are sulfonated very readily (for example, by concentrated sulfuric acid at room temperature). The reactions generate heat. Phenols are also nitrated very rapidly, even by dilute nitric acid. Lactones react similarly to esters. Esters react with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Heat is also generated by the interaction of esters with caustic solutions. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides.
Fire Hazard
Flash point data for 5,7-Dihydroxy-4-methylcoumarin are not available. 5,7-Dihydroxy-4-methylcoumarin is probably combustible.
Properties of 5,7-Dihydroxy-4-methylcoumarin
| Melting point: | 296-299 °C (lit.) |
| Boiling point: | 472.1±14.0 °C(Predicted) |
| Density | 1.456±0.06 g/cm3(Predicted) |
| storage temp. | -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 7.29±0.20(Predicted) |
| color | Off-White to Pale Green |
| λmax | 322nm(MeOH)(lit.) |
| Sensitive | Light Sensitive |
| BRN | 179133 |
| CAS DataBase Reference | 2107-76-8(CAS DataBase Reference) |
| EPA Substance Registry System | 5,7-Dihydroxy-4-methylcoumarin (2107-76-8) |
Safety information for 5,7-Dihydroxy-4-methylcoumarin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for 5,7-Dihydroxy-4-methylcoumarin
| InChIKey | QNVWGEJMXOQQPM-UHFFFAOYSA-N |
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2107-76-8 5,7- Di hydroxy -4- Methyl coumarin 98%View Details
2107-76-8 5,7- Di hydroxy -4- Methyl coumarin 98%View Details
2107-76-8 -
 2107-76-8 99%View Details
2107-76-8 99%View Details
2107-76-8 -
 5,7-Dihydroxy-4-methylcoumarin CAS 2107-76-8View Details
5,7-Dihydroxy-4-methylcoumarin CAS 2107-76-8View Details
2107-76-8 -
 5,7-Dihydroxy-4-methylcoumarin CAS 2107-76-8View Details
5,7-Dihydroxy-4-methylcoumarin CAS 2107-76-8View Details
2107-76-8 -
 5,7-Dihydroxy-4-methylcoumarin CAS 2107-76-8View Details
5,7-Dihydroxy-4-methylcoumarin CAS 2107-76-8View Details
2107-76-8 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0