5,7-Dihydrox -4'-methoxyisoflavone
Synonym(s):4′-Methylgenistein;5,7-Dihydroxy-4′-methoxyisoflavone;Genistein 4′-methyl ether;Olmelin
- CAS NO.:491-80-5
- Empirical Formula: C16H12O5
- Molecular Weight: 284.26
- MDL number: MFCD00006839
- EINECS: 207-744-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
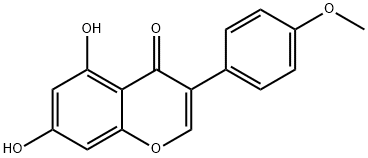
What is 5,7-Dihydrox -4'-methoxyisoflavone?
Description
Biochanin A is a natural isoflavone with diverse biological actions, most notably as a phytoestrogen. It can affect hormone levels by inhibiting 5α-reductase and 17β-hydroxysteroid dehydrogenase or altering aromatase (CYP19A1) activity. Also known as 4’-methyl genistein, biochanin A can be metabolized in vivo to genistein , another phytoestrogen with diverse effects. Biochanin A also intersects with signaling through peroxisome proliferator-activated receptors (PPARs), as it activates PPARγ (EC50 = 19 μM) and has also been shown to activate a PPARα promoter. Moreover, it increases the expression of the PPARγ coactivator PGC-1α, promoting mitochondrial biogenesis. Biochanin A also inhibits fatty acid amide hydrolase (IC50 = 2.4 μM) and acts as an agonist of the aryl hydrocarbon receptor (EC50 = 0.25 μM).
Chemical properties
Off-White Solid
The Uses of 5,7-Dihydrox -4'-methoxyisoflavone
An isoflavone with anticancer proliferation, differentiation and chemopreventitive properties. Inhibits metabolic activation of benzo[a]pyrene
The Uses of 5,7-Dihydrox -4'-methoxyisoflavone
phytoestrogen
The Uses of 5,7-Dihydrox -4'-methoxyisoflavone
It has putative benefits in dietary cancer prophylaxis. It has also been found to inhibit fatty acid amide hydrolase and to act as agonist of PPARgamma, nuclear receptor that is current pharmacological target for the treatment of diabetes type 2. It acts as an antineoplastic agent. It is a selective agonist at ER-β estrogen receptors, and may have chemopreventive efficacy against breast cancer. In line with its low activity at ER-α estrogen receptors, it is essentially devoid of uterotrophic activity. Biochanin A is also a ligand for the aryl hydrocarbon receptor (AhR). It reduces arterial resistance and enhances microcirculation perhaps via effects on potassium and/or calcium ion channels. Induction of sulfotransferases for xenobiotic detoxification has been proposed as a mechanism of its cancer preventive effects. It is a nitric oxide synthase inhibitor and apoptosis inducer
What are the applications of Application
Biochanin A is a nitric oxide synthase inhibitor and apoptosis inducer
Definition
ChEBI: Biochanin A is a member of the class of 7-hydroxyisoflavones that is 7-hydroxyisoflavone which is substituted by an additional hydroxy group at position 5 and a methoxy group at position 4'. A phytoestrogen, it has putative benefits in dietary cancer prophylaxis. It has a role as a phytoestrogen, a plant metabolite, an EC 3.5.1.99 (fatty acid amide hydrolase) inhibitor, a tyrosine kinase inhibitor and an antineoplastic agent. It is a member of 7-hydroxyisoflavones and a member of 4'-methoxyisoflavones. It is a conjugate acid of a biochanin A(1-).
General Description
Biochanin A (BCA) is synthesized in vitro from phloroglucinol. A series of steps involving Friedel?Crafts reaction results in an intermediate product 1-(2,4,6-trihydroxyphenyl)-2-(4 methoxyphenyl) ethanone, which post cyclization leads to BCA. It is catabolized to isoflavone genistein post ingestion.
Biochem/physiol Actions
Biochanin A is an isoflavone phytoestrogen found in red clover (Trifolium pratense) that is a selective agonist at ER-β estrogen receptors, and may have chemopreventive efficacy against breast cancer. In line with its low activity at ER-α estrogen receptors, it is essentially devoid of uterotrophic activity. Biochanin A is also a ligand for the aryl hydrocarbon receptor (AhR). It reduces arterial resistance and enhances microcirculation perhaps via effects on potassium and/or calcium ion channels. Induction of sulfotransferases for xenobiotic detoxification has been proposed as a mechanism of its cancer preventive effects.
Properties of 5,7-Dihydrox -4'-methoxyisoflavone
| Melting point: | 210-213 °C(lit.) |
| Boiling point: | 340-355 °C(Press: 0.5 Torr) |
| Density | 1.420±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | acetone: 10 mg/mL, clear, brown |
| form | Powder |
| pka | 6.50±0.20(Predicted) |
| color | Off-White to Beige |
| Water Solubility | Soluble in water (<1 mg/ml at 25°C), chloroform, methanol, DMSO (57 mg/ml at 25°C), and ethanol (9 mg/ml at 25°C). |
| λmax | 263nm(EtOH)(lit.) |
| BRN | 278107 |
| CAS DataBase Reference | 491-80-5(CAS DataBase Reference) |
| EPA Substance Registry System | 4H-1-Benzopyran-4-one, 5,7-dihydroxy-3-(4-methoxyphenyl)- (491-80-5) |
Safety information for 5,7-Dihydrox -4'-methoxyisoflavone
Computed Descriptors for 5,7-Dihydrox -4'-methoxyisoflavone
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 491-80-5 5,7- Dihydroxy - 4'- methoxyIsoflovone (or) Biochanin-A 98%View Details
491-80-5 5,7- Dihydroxy - 4'- methoxyIsoflovone (or) Biochanin-A 98%View Details
491-80-5 -
 5,7-Dihydroxy-4'-methoxyisoflavone CAS 491-80-5View Details
5,7-Dihydroxy-4'-methoxyisoflavone CAS 491-80-5View Details
491-80-5 -
 5,7-Dihydroxy-4'-methoxyisoflavone CAS 491-80-5View Details
5,7-Dihydroxy-4'-methoxyisoflavone CAS 491-80-5View Details
491-80-5 -
 5,7-Dihydroxy-4'-methoxyisoflavone CAS 491-80-5View Details
5,7-Dihydroxy-4'-methoxyisoflavone CAS 491-80-5View Details
491-80-5 -
 Biochanin A CAS 491-80-5View Details
Biochanin A CAS 491-80-5View Details
491-80-5 -
 Biochanin A CAS 491-80-5View Details
Biochanin A CAS 491-80-5View Details
491-80-5 -
 Biochanin a 98% CAS 491-80-5View Details
Biochanin a 98% CAS 491-80-5View Details
491-80-5 -
 Biochanin A 98% (HPLC) CAS 491-80-5View Details
Biochanin A 98% (HPLC) CAS 491-80-5View Details
491-80-5