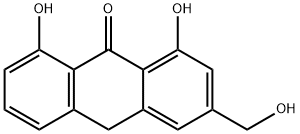
Aloe emodin
Synonym(s):1,8-Dihydroxy 3-hydroxymethylanthraquinone;1,8-Dihydroxy-3-(hydroxymethyl)anthraquinone;3-Hydroxymethylchrysazine;Aloe-emodin;Rhabarberone
- CAS NO.:481-72-1
- Empirical Formula: C15H10O5
- Molecular Weight: 270.24
- MDL number: MFCD00017373
- EINECS: 207-571-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
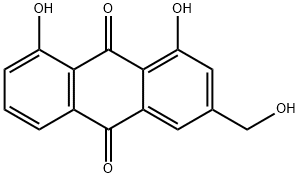
What is Aloe emodin?
Chemical properties
Orange Solid
The Uses of Aloe emodin
Shows significant inhibitory activity against the P-388 leukemia in mice when administered as a suspension in acetone-Tween 80. Has a specific in vitro and in vivo antineuroectodermal tumor activity. Cathartic.
The Uses of Aloe emodin
Aloe-emodin has been used as a standard in high performance liquid chromatography (HPLC) for separation and identification of phenolic compounds in Aloe arborescens Miller var. natalensis Berger (Kidachi aloe).
The Uses of Aloe emodin
Aloe-emodin may be used as an analytical reference standard for the determination of the analyte in plant and animal samples by high-performance liquid chromatography technique.
What are the applications of Application
Aloe-emodin is an inhibitor against the P-388 leukemia in mice
Definition
ChEBI: A dihydroxyanthraquinone that is chrysazin carrying a hydroxymethyl group at position 3. It has been isolated from plant species of the genus Aloe.
General Description
Diacerein is known to be a suitable drug to treat osteoarthritis and prevent vascular diseases. It is a semi-synthetic anthraquinone derivative which acts by inhibiting the production of interleukin-1 and secretion of metalloproteases.
Biochem/physiol Actions
Laxative/cathartic compound; increases the contraction of intestinal smooth muscle by releasing endogenous acetylcholine. Anti-tumor activity is associated with an increased production of reactive oxygen species (ROS) that, in turn, reduces the mitochondrial transmembrane electrical potential, thus inducing a permeability transition that sets in motion a series of events culminating in cellular apoptosis. Genotoxicity and mutagenicity appear to be due to the inhibition of topoisomerase II activity by aloe emodin.
Properties of Aloe emodin
| Melting point: | 223-224°C |
| Boiling point: | 373.35°C (rough estimate) |
| Density | 1.3280 (rough estimate) |
| refractive index | 1.5000 (estimate) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly, Heated) |
| form | neat |
| pka | 6.30±0.20(Predicted) |
| form | Solid |
| color | Orange to Dark Orange |
| Merck | 14,306 |
| BRN | 2059062 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 481-72-1(CAS DataBase Reference) |
Safety information for Aloe emodin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Aloe emodin
| InChIKey | YDQWDHRMZQUTBA-UHFFFAOYSA-N |
Aloe emodin manufacturer
New Products
1-Boc-4-cyanopiperidine tert-Butyl carbazate 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE TETRABUTYLAMMONIUM CYANIDE TETRAHYDRO-2H-PYRAN-3-OL 3-Pyridineacrylic acid Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% Tadalafil Clopidogrel bisulfate Sitagliptin Phosphate Monohydrate Cabergoline Fexofinadine HCl Etoricoxib 4-Amino Acetophenone 2-Chloro Acetophenone Amlodipine Base 2,3,5-Triiodobenzoic Acid Pyrrolidine Diiodo PentoxideRelated products of tetrahydrofuran



You may like
-
 Aloe Emodin CAS 481-72-1View Details
Aloe Emodin CAS 481-72-1View Details
481-72-1 -
 Aloe-emodin CAS 481-72-1View Details
Aloe-emodin CAS 481-72-1View Details
481-72-1 -
 Aloe-emodin CAS 481-72-1View Details
Aloe-emodin CAS 481-72-1View Details
481-72-1 -
 Aloe-emodin 98.00% CAS 481-72-1View Details
Aloe-emodin 98.00% CAS 481-72-1View Details
481-72-1 -
 Aloe-emodin, puriss 95%+ CAS 481-72-1View Details
Aloe-emodin, puriss 95%+ CAS 481-72-1View Details
481-72-1 -
 Aloe-emodin, 90% CAS 481-72-1View Details
Aloe-emodin, 90% CAS 481-72-1View Details
481-72-1 -
 Aloe-emodin >98% (HPLC) CAS 481-72-1View Details
Aloe-emodin >98% (HPLC) CAS 481-72-1View Details
481-72-1 -
 Aloe-emodin CAS 481-72-1View Details
Aloe-emodin CAS 481-72-1View Details
481-72-1
