Ancitabine
- CAS NO.:31698-14-3
- Empirical Formula: C9H11N3O4
- Molecular Weight: 225.2
- MDL number: MFCD00137301
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-11 20:33:26
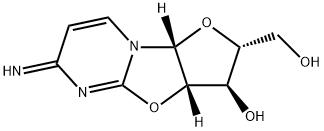
What is Ancitabine?
Originator
Cyclo-C,Kohjin,Japan,1975
The Uses of Ancitabine
Ancitabine is an antineoplastic. Also, it is an agent demonstrating strong synergistic interaction with irofulven, which possesses antitumor activity against solid tumors.
What are the applications of Application
Ancitabine is an antineoplastic
Definition
ChEBI: An organic heterotricyclic compound resulting from the formal condensation of the oxo group of cytidine to the 2' position with loss of water to give the corresponding cyclic ether. A prodrug, it is metabolised to the antineoplastic agent cytarabine, so is used to maintain a more constant antineoplastic action.
Manufacturing Process
A series of reaction steps may be employed in which: (1) Uridine is reacted
with trityl chloride to give 5'-o-trityluridine; (2) Imidazole is reacted with
thiophosgene and that product reacted with the 5'-o-trityluridine to give 2,2'-
anhydro-1-(5'-o-trityl-β-D-arabinofuranosyl)uracil; (3) The preceding uracil
product is converted to the thiouracil using hydrogen sulfide; (4) The trityl
group is removed by treatment with 80% acetic acid; (5) A triacetylated
product is obtained using acetic anhydride; (6) A dithiouracil is prepared from
the uracil intermediate using phosphate pentasulfide.
Preparation of 1-(β-D-arabinofuranosyl)-2-thiocytosine: A solution of 2.0 g of
1-(2',3',5'-O-triacetyl-β-D-arabinofuranosyl)-2,4-dithiouracilin 100 ml of
methanol is saturated with anhydrous ammonia at 0°C. The mixture, in a
glass liner, is heated in a pressure bomb at 100°C for three hours. The
reaction mixture is concentrated to a gum in vacuum, and most of the
byproduct acetamide is removed by sublimation at 60°C/0.1 mm. The residue
is chromatographed on 100 g of silica gel. Elution of the column with
methylene chloride-methanol mixtures with methanol concentrations of 2-25%
gives fractions containing acetamide and a series of brown gums. The desired
product is eluted with 30% methanol-methylene chloride to give a total yield
of 0.386 g (30%), MP 175-180°C (dec.). Recrystallization from methanolisopropanol
furnishes an analytical sample, MP 180-182°C (dec.).
To a solution of 80 mg of 1-(β-D-arabinofuranosyl)-2-thiocytosine in 12 ml of
water is added dropwise 3 ml of a 1 M bromine solution in carbon
tetrachloride. At this point the color of the bromine persists for about 2-3
minutes after each addition. The unreacted bromine is blown off with a stream
of nitrogen, and the reaction mixture is concentrated to a syrup in vacuum
using a bath temperature less than 50°C. The residue is evaporated three
times with 10 ml portions of ethanol, whereupon it crystallizes. The product is
triturated with cold ethanol and with ether to obtain 17 mg of 2,2'-anhydro-1-
(β-D-arabinofuranosyl)cytosine hydrobromide, MP 240°C (dec.).
Treatment of the hydrobromide with a slight excess of ethanolic ammonia
yields the base which may then be converted to the hydrochloride.
Therapeutic Function
Antineoplastic
Properties of Ancitabine
| Melting point: | 269-270 °C (dec.)(lit.) |
| Boiling point: | 366.7°C (rough estimate) |
| Density | 1.3588 (rough estimate) |
| refractive index | 1.5100 (estimate) |
| storage temp. | −20°C |
| pka | 12.67±0.40(Predicted) |
Safety information for Ancitabine
Computed Descriptors for Ancitabine
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1