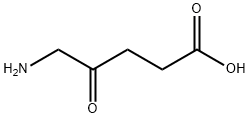
5-Aminolevulinic acid hydrochloride
Synonym(s):ALA;5-Aminolevulinic acid hydrochloride;5-Amino-4-oxopentanoic acid hydrochloride;5-Aminolaevulinic acid hydrochloride;δ-Aminolevulinic acid hydrochloride
- CAS NO.:5451-09-2
- Empirical Formula: C5H10ClNO3
- Molecular Weight: 167.59
- MDL number: MFCD00012869
- EINECS: 226-679-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31
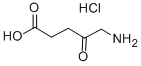
What is 5-Aminolevulinic acid hydrochloride?
Description
5-Aminolevulinic acid hydrochloride (5-ALA) is a naturally occurring amino acid that is an intermediate in the biosynthesis of chlorophyll and haemoglobin. It has anti-inflammatory, antioxidant and antitumour activities, and 5-Aminolevulinic acid hydrochloride-mediated sonodynamic therapy (SDT) has antitumour effects on pancreatic cancer cells. In addition, it is a visualising agent that causes high-grade gliomas to fluoresce under blue light, which can help guide surgeons in removing tumours and is considered a useful imaging tool for brain tumour resection.
Chemical properties
white to pale yellow crystals or
Originator
Levulan Kerastick,DUSA Pharmaceuticals Inc.
The Uses of 5-Aminolevulinic acid hydrochloride
5-Aminolevulinic acid hydrochloride finds an important role as a precursor in the synthesis of tetrapyrroles such as chlorophyll and heme. It is widely utilized in photodynamic therapy of diseases namely, Paget?s disease and human papillomavirus (HPV) infection-associated cervical condylomata acuminata.
The Uses of 5-Aminolevulinic acid hydrochloride
Naturally occurring amino acid; precursor of tetrapyrroles in the biosynthesis of chlorophyll and heme. Antineoplastic (photosensitizer).
The Uses of 5-Aminolevulinic acid hydrochloride
5-Aminolevulinic acid hydrochloride has been used as a supplement for culturing Escherichia coli cells for heme biosynthesis.
What are the applications of Application
5-Aminolevulinic Acid Hydrochloride Salt is a naturally occurring amino acid and precursor of tetrapyrroles.
Definition
ChEBI: A hydrochloride that is the monohydrochloride of 5-aminolevulinic acid. It is metabolised to protoporphyrin IX, a photoactive compound which accumulates in the skin. Used in combination with blue light illumination for the treatment of minimally to moderat ly thick actinic keratosis of the face or scalp.
What are the applications of Application
5-Aminolevulinic acid hydrochloride has been used to activate or inhibit heme biosynthesis in HeLa and K562 cell lines. It has also been used in photodynamic therapy in mice with A431 tumours. Clinical oral administration of 5-Aminolevulinic acid hydrochloride potentiates the antihypertensive effects associated with anaesthetics and is used in the treatment of hypertensive patients, particularly in elderly hypertensive patients receiving antihypertensive medications[1-2].
Manufacturing Process
1) Oxidation Step
2.27 g (10.0 mmol) of N-furfurylphthalimide was charged into a three-necked
glass flask equipped with an oxygen feed tube, a thermometer, and a reflux
condenser, and dissolved in 100 ml of anhydrous pyridine. After the addition
of 7.0 mg of Rose Bengal, oxygen gas was fed at a rate of 20 ml/min at 10°-
20°C under irradiation by light. A 27 W white fluorescent lamp was used as a
light source and the radiation was performed from the outside of the flask.
After 7 hours, the irradiation was terminated and the pyridine was evaporated
under reduced pressure to obtain 2.47 g of a light brown, semi-crystalline
product.
2) Reduction Step (Hydrogenation)
2.00 g of the semi-crystalline solid obtained in (1) was dissolved in 40 ml of
methanol and stirred at 50°C in a hydrogen atmosphere under atmospheric
pressure in the presence of 200 mg of 5% palladium-on-carbon catalyst.
After five hours, the reaction was terminated and the mixture was allowed to
cool to room temperature. The catalyst was removed by filtration and
methanol was evaporated to obtain 2.11 g of white crystals.
The crystals were identified to be 5-phthalimidolevulinic acid by NMR analysis.
The yield was 97%.
3) Hydrolysis Step
100 ml of 6 N hydrochloric acid was added to 2.11 g of the white crystals (2),
and the mixture was heated under reflux for 5 hours.
After evaporating the hydrochloric acid under reduced pressure, a brown solid
product was obtained and dissolved in ethanol. Acetone was added to the
solution and the crystals produced were collected by filtration to obtain 0.689
g of 5-aminolevulinic acid hydrochloride. The yield based on Nfurfurylphthalimide
was 51%.
NMR spectrum data conformed to 5-aminolevulinic acid hydrochloride
Therapeutic Function
Photosensitizer
Biochem/physiol Actions
5-Aminolevulinic acid (5-ALA) is an intermediate in heme biosynthesis and is useful in cancer treatment. It is a non-protein amino acid. 5-ALA also has applications in the field of agriculture. It is being studied as an inducing reagent for protoporphyrin IX (PPIX) dependent fluorescence diagnosis of metastatic lymph nodes. 5-ALA is used for photodynamic therapy of diseases, such as Paget′s disease and HPV infection-associated cervical condylomata acuminata.
Purification Methods
Dry ALA-HCl in a vacuum desiccator over P2O5 overnight, then crystallise it by dissolving it in cold EtOH and adding dry Et2O. Also crystallis
References
[1] Oral Aminolevulinic Acid Hydrochloride[J]. Definitions, 2020. DOI:10.32388/vh56ni.
[2] NOBORU FUKUDA . 5-Aminolevulinic acid hydrochloride enhances bupivacaine-induced hypotension in spontaneously hypertensive rats[J]. Journal of pharmacological sciences, 2023. DOI:10.1016/j.jphs.2023.02.007.
Properties of 5-Aminolevulinic acid hydrochloride
| Melting point: | ~150 °C (dec.) |
| Flash point: | 155-157°C |
| storage temp. | -20°C |
| solubility | H2O: 50 mg/mL |
| form | powder |
| color | White to pale yellow |
| PH | pH (10g/l, 25℃) : 2.5~3.0 |
| Water Solubility | Soluble in dimethyl sulfoxide, methanol and water. |
| Sensitive | Hygroscopic |
| Decomposition | 155-157 ºC |
| Merck | 14,446 |
| BRN | 3690651 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 5451-09-2(CAS DataBase Reference) |
Safety information for 5-Aminolevulinic acid hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for 5-Aminolevulinic acid hydrochloride
| InChIKey | ZLHFONARZHCSET-UHFFFAOYSA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 5-Aminolevulinic acid hydrochloride CAS 5451-09-2View Details
5-Aminolevulinic acid hydrochloride CAS 5451-09-2View Details
5451-09-2 -
 5-Aminolevulinic acid hydrochloride, ≥98% CAS 5451-09-2View Details
5-Aminolevulinic acid hydrochloride, ≥98% CAS 5451-09-2View Details
5451-09-2 -
 5-Aminolevulinic Acid Hydrochloride CAS 5451-09-2View Details
5-Aminolevulinic Acid Hydrochloride CAS 5451-09-2View Details
5451-09-2 -
 Aminolevulinic acid hydrochloride CAS 5451-09-2View Details
Aminolevulinic acid hydrochloride CAS 5451-09-2View Details
5451-09-2 -
 5-Aminolevulinic acid hydrochloride CAS 5451-09-2View Details
5-Aminolevulinic acid hydrochloride CAS 5451-09-2View Details
5451-09-2 -
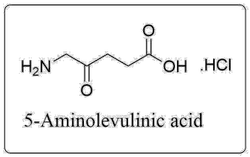 5-Aminolevulinic Acid HCLView Details
5-Aminolevulinic Acid HCLView Details
5451-09-2 -
 5-aminolevulinic Acid Hydrochloride Cas No 5451-09-2View Details
5-aminolevulinic Acid Hydrochloride Cas No 5451-09-2View Details
5451-09-2 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
