5-Aminolevulinic acid
- CAS NO.:106-60-5
- Empirical Formula: C5H9NO3
- Molecular Weight: 131.13
- MDL number: MFCD00044485
- EINECS: 203-414-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-21 22:16:43
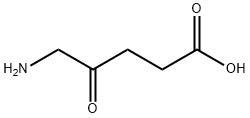
What is 5-Aminolevulinic acid?
Absorption
Oral bioavailability is 50-60%.
Pharmacokinetics (PK) of aminolevulinic acid (ALA) and PpIX was evaluated in a trial of 12 adult subjects with mild to moderate AK with at least 10 AK lesions on the face or forehead. A single dose of one entire tube of ALA (2 grams) was applied under occlusion for 3 hours followed by photodynamic therapy (PDT) to a total area of 20 cm2. The mean ± SD baseline plasma ALA and PpIX concentrations were 20.16 ± 16.53 ng/mL and 3.27 ± 2.40 ng/mL, respectively. In most subjects, an up to 2.5-fold increase of ALA plasma concentrations was observed during the first 3 hours after ALA application. The mean ± SD area under the concentration time curve (AUC0-t) and maximum concentration (Cmax) for baseline corrected ALA (n=12) were 142.83 ± 75.50 ng.h/mL and 27.19 ± 20.02 ng/mL, respectively. The median Tmax (time at which Cmax occurred) was 3 hours.
Two human pharmacokinetic (PK) studies were conducted in subjects with minimally to moderately thick actinic keratoses on the upper extremities, having at least 6 lesions on one upper extremity and at least 12 lesions on the other upper extremity. A single dose comprising of two topical applications of ALA topical solution (each containing 354 mg ALA HCl) were directly applied to the lesions and occluded for 3 hours prior to light treatment.
The first PK study was conducted in 29 subjects and PK parameters of ALA were assessed. The baseline corrected mean ± SD of the maximum concentration (Cmax) of ALA was 249.9 ± 694.5 ng/mL and the median Tmax was 2 hours post dose. The mean ± SD exposure to ALA, as expressed by area under the concentration time curve (AUCt) was 669.9 ± 1610 ng·hr/mL. The mean ± SD elimination half-life (t1/2) of ALA was 5.7 ± 3.9 hours.
A second PK study was conducted in 14 subjects and PK parameters of ALA and PpIX were measured. The baseline corrected PpIX concentrations were negative in at least 50% of samples in 50% (7/14) subjects and AUC could not be estimated reliably. The baseline-corrected mean ± SD of Cmax for ALA and PpIX was 95.6 ± 120.6 ng/mL and 0.95 ± 0.71 ng/mL, respectively. The median Tmax of ALA and PpIX was 2 hours post dose and 12 hours post dose, respectively. The mean AUCt of ALA was 261.1 ± 229.3 ng·hr/mL. The mean ± SD t1/2 of ALA was 8.5 ± 6.7 hours.
In 12 healthy subjects, the absolute bioavailability of ALA following the recommended dose of ALA solution was 100.0% + 1.1 with a range of 78.5% to 131.2%. Maximum ALA plasma
concentrations were reached with a median of 0.8 hour (range 0.5 – 1.0 hour).
Toxicity
There are no available human data on aminolevulinic acid (ALA) in pregnant women to inform a drug-associated risk of adverse developmental outcomes. In animal reproduction studies, no adverse developmental effects were observed with oral ALA HCl administration to pregnant rabbits during organogenesis at doses 3 times the maximum recommended human oral dose.
No carcinogenicity testing has been carried out using ALA. No evidence of mutagenic effects was seen in four studies conducted with ALA to evaluate this potential. In the Salmonella-Escherichia coli/mammalian microsome reverse mutation assay (Ames mutagenicity assay), no increases in the number of revertants were observed with any of the tester strains. In the Salmonella-Escherichia coli/mammalian microsome reverse mutation assay in the presence of solar light radiation (Ames mutagenicity assay with light), ALA did not cause an increase in the number of revertants per plate of any of the tester strains in the presence or absence of simulated solar light. In the L5178Y TK± mouse lymphoma forward mutation assay, ALA was evaluated as negative with and without metabolic activation under the study conditions. PpIX formation was not demonstrated in any of these in vitro studies. In the in vivo mouse micronucleus assay, ALA was considered negative under the study exposure conditions. In contrast, at least one report in the literature has noted genotoxic effects in cultured rat hepatocytes after ALA exposure with PpIX formation. Other studies have documented oxidative DNA damage in vivo and in vitro as a result of ALA exposure.
No assessment of effects of ALA HCl on fertility has been performed in laboratory animals. It is unknown what effects systemic exposure to ALA HCl might have on fertility or reproductive function.
Description
5-aminolevulinic acid is the simplest delta-amino acid in which the hydrogens at the gamma position are replaced by an oxo group. It is metabolised to protoporphyrin IX, a photoactive compound which accumulates in the skin. Used (in the form of the hydrochloride salt)in combination with blue light illumination for the treatment of minimally to moderately thick actinic keratosis of the face or scalp. It has a role as a photosensitizing agent, an antineoplastic agent, a dermatologic drug, a prodrug, a plant metabolite, a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. It is a delta-amino acid and a 4-oxo monocarboxylic acid. It derives from a 4-oxopentanoic acid. It is a conjugate base of a 5-ammoniolevulinic acid. It is a conjugate acid of a 5-aminolevulinate. It is a tautomer of a 5-ammoniolevulinate.
Originator
Levulan Kerastick,DUSA Pharmaceuticals Inc.
The Uses of 5-Aminolevulinic acid
5-Aminolevulinic acid (ALA), a nonprotein amino acid involved in tetrapyrrole synthesis, has been widely applied in agriculture, medicine, and food production.
5-Aminolevulinic acid (5-ALA) is an intermediate in heme biosynthesis and is useful in cancer treatment. It is a non-protein amino acid. 5-ALA also has applications in the field of agriculture. It is being studied as an inducing reagent for protoporphyrin IX (PPIX) dependent fluorescence diagnosis of metastatic lymph nodes. 5-ALA is used for photodynamic therapy of diseases, such as Paget′s disease and HPV infection-associated cervical condylomata acuminata.
Intermediate in heme biosynthesis.
Indications
As a topical gel, aminolevulinic acid (ALA) is indicated for lesion-directed and field-directed treatment of actinic keratoses (AKs) of mild-to-moderate severity on the face and scalp in combination with photodynamic therapy (PDT) using BF-RhodoLED? lamp, a narrowband, red light illumination source. As a topical solution, ALA can also be used for the same indication mentioned previously in addition to AKs of the upper extremities, but in conjunction with blue light illumination using the BLU-U Blue Light Photodynamic Therapy Illuminator. Finally, ALA is also available as an oral solution to be used as an adjunct for the visualization of glioma during surgery.
Background
A compound produced from succinyl-CoA and glycine as an intermediate in heme synthesis. It is used as a photochemotherapy for actinic keratosis. [PubChem]
Indications
Aminolevulinic acid (ALA HCl, Levulan Kerastick) is indicated for the treatment of nonhyperkeratotic actinic keratosis of the face and scalp. It has two components, an alcohol solution vehicle and ALA HCl as a dry solid. The two are mixed prior to application to the skin. When applied to human skin, ALA is metabolized to protoporphyrin, which accumulates and on exposure to visible light produces a photodynamic reaction that generates reactive oxygen species (ROS).The ROS produce cytotoxic effects that may explain therapeutic efficacy. Local burning and stinging of treated areas of skin due to photosensitization can occur.
Definition
ChEBI: 5-aminolevulinic acid is the simplest delta-amino acid in which the hydrogens at the gamma position are replaced by an oxo group. It is metabolised to protoporphyrin IX, a photoactive compound which accumulates in the skin. Used (in the form of the hydrochloride salt)in combination with blue light illumination for the treatment of minimally to moderately thick actinic keratosis of the face or scalp. It has a role as a photosensitizing agent, an antineoplastic agent, a dermatologic drug, a prodrug, a plant metabolite, a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. It is a delta-amino acid and a 4-oxo monocarboxylic acid. It is functionally related to a 4-oxopentanoic acid. It is a conjugate base of a 5-ammoniolevulinic acid. It is a conjugate acid of a 5-aminolevulinate. It is a tautomer of a 5-ammoniolevulinate.
Manufacturing Process
1) Oxidation Step
2.27 g (10.0 mmol) of N-furfurylphthalimide was charged into a three-necked
glass flask equipped with an oxygen feed tube, a thermometer, and a reflux
condenser, and dissolved in 100 ml of anhydrous pyridine. After the addition
of 7.0 mg of Rose Bengal, oxygen gas was fed at a rate of 20 ml/min at 10°-
20°C under irradiation by light. A 27 W white fluorescent lamp was used as a
light source and the radiation was performed from the outside of the flask.
After 7 hours, the irradiation was terminated and the pyridine was evaporated
under reduced pressure to obtain 2.47 g of a light brown, semi-crystalline
product.
2) Reduction Step (Hydrogenation)
2.00 g of the semi-crystalline solid obtained in (1) was dissolved in 40 ml of
methanol and stirred at 50°C in a hydrogen atmosphere under atmospheric
pressure in the presence of 200 mg of 5% palladium-on-carbon catalyst.
After five hours, the reaction was terminated and the mixture was allowed to
cool to room temperature. The catalyst was removed by filtration and
methanol was evaporated to obtain 2.11 g of white crystals.
The crystals were identified to be 5-phthalimidolevulinic acid by NMR analysis.
The yield was 97%.
3) Hydrolysis Step
100 ml of 6 N hydrochloric acid was added to 2.11 g of the white crystals (2),
and the mixture was heated under reflux for 5 hours.
After evaporating the hydrochloric acid under reduced pressure, a brown solid
product was obtained and dissolved in ethanol. Acetone was added to the
solution and the crystals produced were collected by filtration to obtain 0.689
g of 5-aminolevulinic acid hydrochloride. The yield based on Nfurfurylphthalimide
was 51%.
NMR spectrum data conformed to 5-aminolevulinic acid hydrochloride
Therapeutic Function
Photosensitizer
Biological Activity
5-Aminolevulinic acid (5-ALA) is a precursor in the biosynthesis of porphyrins, including heme. The conversion of 5-ALA to protoporphyrins within tissues produces a photosensitive target that produces reactive oxygen species upon exposure to light.1 In this way, it is used in photodynamic therapy for a range of dermatological conditions, cancers, and other diseases. Also, oral administration of 5-ALA leads to the preferential accumulation of the fluorescent molecule protoporphyrin IX within certain types of cancer cells. This allows fluorescence-based identification of tumor tissue for accurate resection of diseased tissue.
Pharmacokinetics
The metabolism of aminolevulinic acid (ALA) is the first step in the biochemical pathway resulting in heme synthesis. Aminolevulinic acid is not a photosensitizer, but rather a metabolic precursor of protoporphyrin IX (PpIX), which is a photosensitizer. The synthesis of ALA is normally tightly controlled by feedback inhibition of the enzyme, ALA synthetase, presumably by intracellular heme levels. ALA, when provided to the cell, bypasses this control point and results in the accumulation of PpIX, which is converted into heme by ferrochelatase through the addition of iron to the PpIX nucleus.
Enzyme inhibitor
This key metabolic precursor (FW = 131.13 g/mol; CAS 106-60-5; pKa values = 4.05 and 8.90 at 25°C; Symbol: ALA), also known as daminolevulinic acid, is essential for the biosynthesis of metal ion-binding tetrapyrrole ring systems (porphyrins, chlorophylls, and cobalamins). In non-photosynthetic eukaryotes (animals, insects, fungi, protozoa, and alphaproteobacteria), d-aminolevulinic acid is produced by the enzyme ALA synthase, using glycine and succinyl CoA as substrates. In plants, algae, bacteria, and archaea, it is produced from glutamyl-tRNA and glutamate-1-semialdehyde. 5-Aminolevulinic acid inhibits (R)-3-amino-2- methylpropionate:pyruvate aminotransferase. ALA Phototherapy: Protoporphyrin IX, the immediate heme precursor is a highly effective tissue photosensitizer that is synthesized in four steps from 5- aminolevulinic acid. ALA synthesis is regulated via a feedback inhibition and gene repression mechanism linked to the concentration of free heme. In certain cell and tissue types, addition of exogenous ALA bypasses these regulation mechanisms, inducing uptake and synthesis of photosensitizing concentrations of Protoporphyrin IX, or PpIX. Topical application of ALA to certain malignant and non-malignant skin lesions, for example, can induce a clinically useful degree of lesion-specific photosensitization (e.g., superficial basal cell carcinomas show high response rate (~79%) after a single phototherapy treatment). ALA also induces localized tissue-specific photosensitization, when injected intradermally. In this sense, ALA and its methyl ester (methyl aminolevulinate, or MAL; trade name: Metvix?) are prodrugs that increase the amounts of the active drug (PpIX).
Metabolism
Exogenous aminolevulinic acid (ALA) is metabolized to PpIX, but the fraction of administered ALA that is metabolized to PpIX is unknown. The average plasma AUC of PpIX is less than 6% of that of ALA.
Properties of 5-Aminolevulinic acid
| Melting point: | 118-119 °C |
| Boiling point: | 242.42°C (rough estimate) |
| Density | 1.3121 (rough estimate) |
| refractive index | 1.4300 (estimate) |
| storage temp. | 2-8°C(protect from light) |
| solubility | Soluble in DMSO |
| pka | 4.05(at 25℃) |
| CAS DataBase Reference | 106-60-5(CAS DataBase Reference) |
| EPA Substance Registry System | Pentanoic acid, 5-amino-4-oxo- (106-60-5) |
Safety information for 5-Aminolevulinic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 5-Aminolevulinic acid
5-Aminolevulinic acid manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
