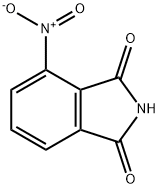
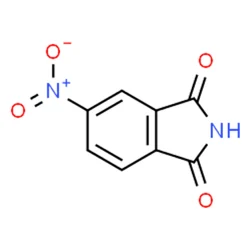
4-Nitrophthalimide
- CAS NO.:89-40-7
- Empirical Formula: C8H4N2O4
- Molecular Weight: 192.13
- MDL number: MFCD00005884
- EINECS: 201-905-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-18 14:22:45
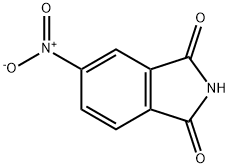
What is 4-Nitrophthalimide?
Chemical properties
slight yellow powder
The Uses of 4-Nitrophthalimide
4-Nitrophthalimide is used as intermediate of organic synthesis and fluorescent dyes, it can be used to produce azo dyes, luminol, etc. It can also be used to prepare 4-nitrophthalonitrile, 5-cyanophthalide and other drug intermediates.
Preparation
4-Nitrophthalimide is synthesized from phthalimide by nitration. Cool the fuming nitric acid to below 5°C, slowly add concentrated sulfuric acid dropwise, and control the temperature to 10-13°C. Plus phthalimide. Leave at room temperature overnight. Then, it was poured into ice water with stirring, and the precipitate was precipitated below 20°C. Filter, wash with water, dry, and then recrystallize from ethanol to obtain 4-nitrophthalimide with a yield of 60%.
General Description
Yellow powder.
Air & Water Reactions
4-Nitrophthalimide can be hydrolyzed. . Insoluble in water.
Reactivity Profile
A nitrated amine. Amines are combustible. The combustion of amines yields noxious NOx. REACTIVITY - Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Aromatic nitro compounds range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, they may begin a vigorous reaction that culminates in a detonation. The aromatic nitro compounds may explode in the presence of a base such as sodium hydroxide or potassium hydroxide even in the presence of water or organic solvents. The explosive tendencies of aromatic nitro compounds are increased by the presence of multiple nitro groups.
Fire Hazard
Flash point data for 4-Nitrophthalimide are not available, however 4-Nitrophthalimide is probably combustible.
Safety Profile
Mutation data reported. Whenheated to decomposition it emits toxic vapors of NOx.
Synthesis
200 mL of sulfuric acid and 50 mL of fuming nitric acid were cooled on an ice
bath and 40 g (0.272 mol) phthalimide was added in portions within 1-1.5 hours so as
not to exceed the internal temperature beyond 10-15 ??C. The mixture was stirred
over ice bath for half an hour and the internal temperature was raised to 35 ??C, and
yellow particles were observed to dissolve. The mixture was stirred for an additional
1 hour, then cooled to 0 ??C again and poured onto 1 kg of ice water mixture. Yellow
4-nitrophthalonitrile precipitated, it was filtered and washed with water until the
filtrate was neutral to blue litmus paper. It was crystallized from 850-900 mL of ethyl
alcohol. Bright yellow crystals were filtered, washed with cold ethyl alcohol, and
dried in a vacuum oven at 80-90 ??C.
Properties of 4-Nitrophthalimide
| Melting point: | 206 °C |
| Boiling point: | 328.09°C (rough estimate) |
| Density | 1.5513 (rough estimate) |
| refractive index | 1.4900 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| form | powder to crystal |
| pka | 7.79±0.20(Predicted) |
| color | Yellow powder |
| Water Solubility | <0.01 g/100 mL at 18 ºC |
| BRN | 180224 |
| Stability: | Stable. Combustible. Incompatible with moisture, water, strong oxidising agents, strong bases. |
| CAS DataBase Reference | 89-40-7(CAS DataBase Reference) |
| NIST Chemistry Reference | 4-Nitro-phthalimide(89-40-7) |
| EPA Substance Registry System | 4-Nitrophthalimide (89-40-7) |
Safety information for 4-Nitrophthalimide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-Nitrophthalimide
| InChIKey | ANYWGXDASKQYAD-UHFFFAOYSA-N |
4-Nitrophthalimide manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Powder 4 Nitrophthalimide, Packaging Type: BagView Details
Powder 4 Nitrophthalimide, Packaging Type: BagView Details
22312-61-4 -
 4-NITRO PHTHALIMIDEView Details
4-NITRO PHTHALIMIDEView Details
89-40-7 -
 4 Nitrophthalimide PowderView Details
4 Nitrophthalimide PowderView Details
89-40-7 -
 4 Nitro PhthalimideView Details
4 Nitro PhthalimideView Details
89-40-7 -
 4 Nitrophthalimide PowderView Details
4 Nitrophthalimide PowderView Details
89-40-7 -
 4 NitrophthalimideView Details
4 NitrophthalimideView Details
89-40-7 -
 4 Nitrophthalimide SolidView Details
4 Nitrophthalimide SolidView Details
20260-16-6 -
 Chemical Grade White 4 Nitrophthalimide PowderView Details
Chemical Grade White 4 Nitrophthalimide PowderView Details
31643-49-9
