4-Nitrophthalic anhydride
- CAS NO.:5466-84-2
- Empirical Formula: C8H3NO5
- Molecular Weight: 193.11
- MDL number: MFCD00005922
- EINECS: 226-776-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-24 18:13:52
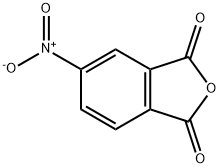
What is 4-Nitrophthalic anhydride?
Chemical properties
off-white to light yellow-brown powder. Soluble in hot alcohol, hot acetic acid and acetone, insoluble in water.
The Uses of 4-Nitrophthalic anhydride
4-Nitrophthalic anhydride is used an an intermediate for the synthesis of a benzimidazole PARP inhibitor I (succinate salt) (ABT-472).
The Uses of 4-Nitrophthalic anhydride
5-Nitrophthalic Anhydride is used in the preparation of phthalic acids as well as compounds with anticonvulsant activities.
Preparation
4-Nitrophthalic anhydride is synthesized by hydrolysis of 4-nitrophthalimide and then dehydration. The 4-nitrophthalimide was added to the sodium hydroxide solution, heated and boiled for 15min. Adjust pH to 6-8 with nitric acid, and add nitric acid to boil for 5 min. Cooling, filtering, the filtrate was extracted with ether, the extract was dried and then the ether was evaporated, that is, 4-Nitrophthalic anhydride crystals were precipitated. Yield 95%.
General Description
Yellow powder.
Air & Water Reactions
Reacts with water. The organic acids produced by this reaction are significantly more soluble in water.
Reactivity Profile
4-Nitrophthalic anhydride reacts exothermically with water. The reactions are usually slow, but might become violent if local heating accelerates their rate. Acids accelerate the reaction with water. Incompatible with acids, strong oxidizing agents, alcohols, amines, and bases.
Fire Hazard
Flash point data for 4-Nitrophthalic anhydride are not available, however 4-Nitrophthalic anhydride is probably combustible.
Purification Methods
Distil the anhydride in a vacuum and/or recrystallise it from *C6H6 or Et2O/pet ether. Dry it in vacuo. It forms addition compounds with anthracene (m 118o), and phenanthrene (m 96o). [Beilstein 17 III/IV 6150, 17/11 V 267.]
Precautions
Moisture Sensitive. Store away from strong bases and oxidizing agents. Incompatible with water, bases, strong acids and oxidizing agents.
Properties of 4-Nitrophthalic anhydride
| Melting point: | 116-120 °C(lit.) |
| Boiling point: | 197 °C / 8mmHg |
| Density | 1.6392 (rough estimate) |
| refractive index | 1.4700 (estimate) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Acetone (Slightly), Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| color | Pale Beige |
| Water Solubility | Hydrolysis |
| Sensitive | Moisture Sensitive |
| BRN | 179682 |
| CAS DataBase Reference | 5466-84-2(CAS DataBase Reference) |
| NIST Chemistry Reference | 4-Nitrophthalic anhydride(5466-84-2) |
| EPA Substance Registry System | 5-Nitrophthalic anhydride (5466-84-2) |
Safety information for 4-Nitrophthalic anhydride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-Nitrophthalic anhydride
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 5466-84-2 4-nitro pthalic anhydride 98%View Details
5466-84-2 4-nitro pthalic anhydride 98%View Details
5466-84-2 -
 4-Nitrophthalic Anhydride CAS 5466-84-2View Details
4-Nitrophthalic Anhydride CAS 5466-84-2View Details
5466-84-2 -
 4-Nitrophthalic anhydride CAS 5466-84-2View Details
4-Nitrophthalic anhydride CAS 5466-84-2View Details
5466-84-2 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
