4-Ethylphenol
- CAS NO.:123-07-9
- Empirical Formula: C8H10O
- Molecular Weight: 122.16
- MDL number: MFCD00002393
- EINECS: 204-598-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 14:18:24
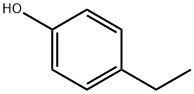
What is 4-Ethylphenol?
Description
4-Ethylphenol (4-EP) is a phenolic compound with the formula C8H10O. It is colorless or white needles with a powerful woody-phenolic and yet somewhat sweet odor and tends to be yellow on exposure to light. It is slightly solouble in water, solouble in alcohol, ether and benzene and carbon disulfide. It is found in arabica coffee and can be produced in wine and beer by the spoilage yeast Brettanomyces. It is the starting material for the production of 4-vinylphenol and of various antioxidants and can also be used as an intermediate for pharmaceuticals and dyes. It is an industrially versatile commodity chemical widely applied in the pharmaceutical and food industries.
Chemical properties
4-Ethylphenol, also known as p-Ethylphenol, is a colorless or white crystalline compound. It can turn yellow when exposed to light. It has a powerful woody-phenolic, medicinal, yet somewhat sweet odor. Its melting point is 4143℃, boiling point is 218219°C, and flash point is 100°C. It has a relative density of 1.011 and a refractive index of 1.5239. It is slightly soluble in water and soluble in ethanol, ether, acetone, benzene, and carbon disulfide. It is a natural component found in foods such as egg fruit, European sour cranberry, pork, rum, whisky, and coffee.
Occurrence
Reported found in yellow passion fruit, smoked fish, lean fish, beer, cognac, rum, whiskey, cider, sherry, grape wines and coffee
The Uses of 4-Ethylphenol
4-Ethylphenol is suitable for use in the determination of 4-ethylguaiacol and 4-ethylphenol in the aroma of red wines using headspace-solid-phase microextraction method. It is a chemical reagent used in the synthesis of 5-HT1A receptor agonists endowed with antinociceptive activity in vivo. It is also used in the preparation of isopropylamides as potent and noncovalent proteosome inhibitors.
Preparation
4-Ethylphenol is prpared by catalytic reaction between phenol and ethylene or ethanol; by heating 8-chloro-3-ethylbenzene with NaOH in the presence of copper powder.
Definition
ChEBI: 4-ethylphenol is a member of the class of phenols carrying an ethyl substituent at position 4. It has a role as a fungal xenobiotic metabolite.
Production Methods
4-Ethylphenol is produced industrially by sulfonation of ethylbenzene under mild (kinetically controlled) conditions and subsequent alkali fusion of the 4-ethylbenzenesulfonic acid obtained. The purity of the commercial product is 98%.
Aroma threshold values
Detection: 42 to 130 ppb
Synthesis Reference(s)
The Journal of Organic Chemistry, 14, p. 1089, 1949 DOI: 10.1021/jo01158a018
General Description
4-Ethylphenol is a volatile phenol. It has been synthesized from p-coumaric acid by employing a synthetic medium containing Dekkera bruxellensis ISA 1791. It has been identified as an aroma compound in red wine.
Hazard
Toxic material.
Safety Profile
Poison by intravenous route. When heated to decomposition it emits acrid smoke and irritating vapors.
Toxicology
4-Ethylphenol is a potentially toxic compound, capable of producing respiratory distress, cardiovascular collapse, shock, ventricular tachycardia, and coma in an adult. Liver, lung, central nervous system and renal injury may also occur. In case of exposure to eyes, irrigate exposed eyes with copious amounts of room temperature water for at least 15 minutes. Monitor for respiratory distress in case of inhalation exposure. Systemic manifestations of toxicity may include nausea, vomiting, diarrhea, dyspnea, tachypnea, pallor, and profuse sweating.
Purification Methods
Non-acidic impurities are removed by passing steam through a boiling solution containing 1 mole of the phenol and 1.75 moles of NaOH (as an aqueous 10% solution). The residue is cooled and acidified with 30% (v/v) H2SO4, and the free phenol is extracted into diethyl ether. The extract is washed with water, dried with CaSO4 and the ether is evaporated. The phenol is distilled at 100mm pressure through a Stedman gauze-packed column (p 11). It is further purified by fractional crystallisation by partial freezing, and by zone refining, under N2 [Biddiscombe et al. J Chem Soc 5764 1963]. Alternatively purify it via the benzoate, as for phenol. The 4-nitrophenylbenzoate has m 80o (from EtOH). [Beilstein 6 H 470, 6 III 1663, 5 IV 3020.]
References
[1] https://en.wikipedia.org/wiki/4-Ethylphenol
[2] http://www.hmdb.ca/metabolites/HMDB29306
[3] https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/f?./temp/~x8ypqu:1:cpp
[4] https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/f?./temp/~x8ypqu:1:manf
[5] YING ZHANG Shaojun D Liangkun Long. Heterologous synthesis of 4-ethylphenol in engineered Escherichia coli[J]. Process Biochemistry, 2020, 96: Pages 157-164. DOI:10.1016/j.procbio.2020.06.003.
[6] WOHLFARTH C. Dielectric constant of 4-ethylphenol[C]. 1900: 0. DOI:10.1007/978-3-540-75506-7\_257.
[7] K H JONES D J H P W Trudgill. 4-Ethylphenol metabolism by Aspergillus fumigatus.[J]. Applied and Environmental Microbiology, 1994, 60 6: 1978-1983. DOI:10.1128/aem.60.6.1978-1983.1994.
Properties of 4-Ethylphenol
| Melting point: | 40-42 °C(lit.) |
| Boiling point: | 218-219 °C(lit.) |
| Density | 1,011 g/cm3 |
| vapor density | 4.2 (vs air) |
| vapor pressure | 0.13 mm Hg ( 20 °C) |
| refractive index | 1.5330 |
| FEMA | 3156 | P-ETHYLPHENOL |
| Flash point: | 213 °F |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | 4.9g/l |
| form | Crystalline Mass or Crystals |
| pka | pK1:10.0 (25°C) |
| Specific Gravity | 1.0123 (20℃) |
| color | White to brownish |
| Odor | at 1.00 % in dipropylene glycol. phenolic castoreum smoke guaiacol |
| Water Solubility | 4.9 g/L (25 ºC) |
| JECFA Number | 694 |
| BRN | 1363317 |
| Stability: | Stable. Incompatible with acid chlorides, acid anhydrides, oxidizing agents. |
| CAS DataBase Reference | 123-07-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Phenol, 4-ethyl-(123-07-9) |
| EPA Substance Registry System | p-Ethylphenol (123-07-9) |
Safety information for 4-Ethylphenol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-Ethylphenol
| InChIKey | HXDOZKJGKXYMEW-UHFFFAOYSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 4-Ethylphenol 123-07-9 99%View Details
4-Ethylphenol 123-07-9 99%View Details
123-07-9 -
 4-Ethylphenol CAS 123-07-9View Details
4-Ethylphenol CAS 123-07-9View Details
123-07-9 -
 4-Ethylphenol CAS 123-07-9View Details
4-Ethylphenol CAS 123-07-9View Details
123-07-9 -
 4-Ethylphenol, 97% CAS 123-07-9View Details
4-Ethylphenol, 97% CAS 123-07-9View Details
123-07-9 -
 4-Ethylphenol CAS 123-07-9View Details
4-Ethylphenol CAS 123-07-9View Details
123-07-9 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4
