4-Chlorophenol
Synonym(s):4-Chloro-1-hydroxybenzene;4-Chlorophenol
- CAS NO.:106-48-9
- Empirical Formula: C6H5ClO
- Molecular Weight: 128.56
- MDL number: MFCD00002318
- EINECS: 203-402-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:41

What is 4-Chlorophenol?
Absorption
absorbed from gastrointestinal tract.
Toxicity
Signs and symptoms in human : When undiluted, it whitens & cauterizes the skin & mucous membranes. 4-chlorophenol was shown to permeate the skin. It produces damage at a threshold concentration of 0.75% (w/v).
Routes of Entry: Absorbed through skin. Eye contact.
Toxicity to Animals:
Acute oral toxicity (LD50): 367 mg/kg [Mouse]. Acute dermal toxicity (LD50): 1500 mg/kg [Rat].
Chronic Effects on Humans:
CARCINOGENIC EFFECTS: Classified 2B (Possible for human.) by IARC. DEVELOPMENTAL TOXICITY: Classified
Reproductive system/toxin/male, Development toxin [POSSIBLE]. May cause damage to the following organs: liver, brain,
gastrointestinal tract, upper respiratory tract, central nervous system (CNS).
Other Toxic Effects on Humans: Very hazardous in case of skin contact (irritant).
Chemical properties
off-white to light tan crystals or powder
Chemical properties
All isomers have a characteristic odor.
The Uses of 4-Chlorophenol
It is used as main raw material of compounding for medicine, dye, plastic and other industries. 4-Chlorophenol was used in visible-light-induced degradation of 4-chlorophenol in aqueous suspension of pure TiO2.
The Uses of 4-Chlorophenol
Intermediates of Liquid Crystals
The Uses of 4-Chlorophenol
antineoplastic
The Uses of 4-Chlorophenol
Biocide; disinfectant for home, hospital and farm.
Indications
Used as an intermediate in organic synthesis of dyes and drugs. Local antibacterial agent in root canal therapy, as topical antiseptic in ointments
Background
P-chlorophenol is a white crystals with a strong phenol odor. Slightly soluble to soluble in water, depending on the isomer, and denser than water. Noncombustible.
Definition
ChEBI: A monochlorophenol substituted at the pare position by a chlorine atom.
Synthesis Reference(s)
The Journal of Organic Chemistry, 50, p. 2145, 1985 DOI: 10.1021/jo00212a029
Tetrahedron Letters, 24, p. 3117, 1983 DOI: 10.1016/S0040-4039(00)88111-3
General Description
White crystals with a strong phenol odor Slightly soluble to soluble in water, depending on the isomer, and denser than water. Noncombustible. Used as an intermediate in organic synthesis of dyes and drugs.
Air & Water Reactions
Slightly soluble to soluble in water.
Reactivity Profile
CHLOROPHENOLS, SOLID are incompatible with acid chlorides, acid anhydrides and oxidizing agents. Also incompatible with iron . Liquefy and darken in color at temperatures above 108°F.
Hazard
Toxic by skin absorption, inhalation, or ingestion; strong irritant to tissue.
Health Hazard
Inhalation causes headache, dizziness, weak pulse. Ingestion causes irritation of mouth and stomach; headache, dizziness, weak pulse. Contact with eyes causes severe irritation and burning. Contact with skin causes irritation and burn; if absorbed, causes same symptoms as inhalation.
Fire Hazard
Special Hazards of Combustion Products: Toxic and irritating hydrogen chloride and chlorine gases may form in fires.
Flammability and Explosibility
Not classified
Safety Profile
Poison by inhalation and intraperitoneal routes. Moderately toxic by ingestion, skin contact, and subcutaneous routes. A severe skin and eye irritant. Human systemic effects by inhalation: excitement, irritability. Mutation data reported. Combustible when exposed to heat or flame. To fight fire, use water, spray, mist, fog, foam, dry chemical. When heated to decomposition it emits toxic fumes of Cl-. See also CHLOROPHENOLS and CHLORIDES.
Potential Exposure
Monochlorophenols are used in the manufacture of fungicides, slimicides, bactericides, pesticides, herbicides, disinfectants, wood and glue preservatives; in the production of phenolic resins; in the extraction of certain minerals from coal; as a denaturant for ethanol; as an antiseptic; as a disinfectant, and others.
Metabolism
P-Chlorophenol yields p-chloroanisole in guinea pigs. P-Chlorophenol yields 4-chlorocatechol p-chloro phenyl-beta-D-glucuronide & p-chlorphenyl sulfate in rabbits. P-Chlorophenol yields p-chlorophenyl sulfate in rats.
Shipping
UN 2020 (solid); UN2021 (liquid) Chlorophenols, solid and liquid, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Distil the phenol, then crystallise it from pet ether (b 40-60o) or hexane, and dry it under vacuum over P2O5 at room temperature. [Bernasconi & Paschalis J Am Chem Soc 108 2969 1986, Beilstein 6 IV 820.]
Incompatibilities
May form explosive mixture with air. Contact with oxidizing agents can cause fire and explosion hazard. Heat produces hydrogen chloride and chlorine. Corrosive to aluminum, copper and other chemically active metals.
Waste Disposal
Incinerate in admixture with flammable solvent in furnace equipped with afterburner and scrubber.
Properties of 4-Chlorophenol
| Melting point: | 42-45 °C |
| Boiling point: | 220 °C (lit.) |
| Density | 1.306 g/mL at 25 °C (lit.) |
| vapor density | 4.43 (vs air) |
| vapor pressure | 1 mm Hg ( 49.8 °C) |
| refractive index | 1.5579 |
| Flash point: | 240 °F |
| storage temp. | 2-8°C |
| solubility | ethanol: soluble50mg/mL, clear to very slightly hazy, colorless to faintly brownish-yellow |
| form | Low Melting Crystalline Mass |
| pka | 9.18(at 25℃) |
| color | White to pale yellow |
| Odor | Phenolic |
| Odor Threshold | 30 ppm |
| Water Solubility | 2.7 g/100 mL (20 ºC) |
| Sensitive | Air Sensitive |
| Merck | 14,2154 |
| BRN | 507004 |
| Dielectric constant | 9.5(54.0℃) |
| Stability: | Stable. Incompatible with acid chlorides, acid anhydrides, oxidizing agents, iron. |
| CAS DataBase Reference | 106-48-9(CAS DataBase Reference) |
| NIST Chemistry Reference | p-Chlorophenol(106-48-9) |
| EPA Substance Registry System | p-Chlorophenol (106-48-9) |
Safety information for 4-Chlorophenol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-Chlorophenol
4-Chlorophenol manufacturer
JSK Chemicals
Antares Chem Private Limited
Classic Technochem Corporation
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 4-Chlorophenol 98%View Details
4-Chlorophenol 98%View Details -
 4-Chlorophenol CASView Details
4-Chlorophenol CASView Details -
 p-Chlorophenol pure CAS 106-48-9View Details
p-Chlorophenol pure CAS 106-48-9View Details
106-48-9 -
 4-Chlorophenol solution CASView Details
4-Chlorophenol solution CASView Details -
 Para Chloro Phenol Chemical, Cas Number: 106-48-9View Details
Para Chloro Phenol Chemical, Cas Number: 106-48-9View Details
106-48-9 -
 Para Chloro Phenol, Purity: 99% Minimum, Packaging Size: 200 Kg DrumView Details
Para Chloro Phenol, Purity: 99% Minimum, Packaging Size: 200 Kg DrumView Details
106-48-9 -
 4-Chlorophenol, For Industrial, Grade Standard: Technical GradeView Details
4-Chlorophenol, For Industrial, Grade Standard: Technical GradeView Details
106-48-9 -
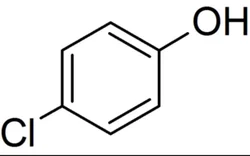 Para Chloro PhenolView Details
Para Chloro PhenolView Details
106-48-9
