2-Bromo-4'-methoxyacetophenone
Synonym(s):ω-Bromo-4-methoxyacetophenone;4-Methoxyphenacyl bromide
- CAS NO.:2632-13-5
- Empirical Formula: C9H9BrO2
- Molecular Weight: 229.07
- MDL number: MFCD00000201
- EINECS: 220-118-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-19 15:05:27
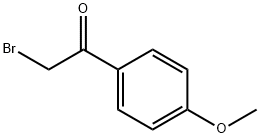
What is 2-Bromo-4'-methoxyacetophenone?
Chemical properties
off-white to light brown crystals or
The Uses of 2-Bromo-4'-methoxyacetophenone
2-Bromo-4'-methoxyacetophenone is a α-haloacetophenone derivatives tested for inhibition of protein tyrosine phosphatases SHP-1 and PTP1B.
The Uses of 2-Bromo-4'-methoxyacetophenone
2-Bromo-4'-methoxyacetophenone, is used as a cell-permeable, covalent and potent protein tyrosine phosphatase inhibitor.
What are the applications of Application
PTP Inhibitor II is a cell-permeable, covalent and potent protein tyrosine phosphatase inhibitor
Preparation
Obtained by reaction of N-bromosuccinimide (NBS) with 4-methoxyacetophenone in the presence of trimethylsilyl trifluoromethanesulfonate (TMS-OTf) in acetonitrile at r.t. for 24 h (87%).
Biological Activity
ki: 128 μmptp inhibitor ii is a protein tyrosine phosphatase (ptp) inhibitor.protein tyrosine phosphatases (ptps) are reported to be involved in the etiology of diabetes mellitus, neural diseases such as alzheimer and parkinson diseases, regulation of allergy and inflammation, or ptps are even regarded to be responsible for the pathogens.
in vitro
in a previous study, it was found that all of the previously reported ptp inhibitors contained a negatively charged, nonhydrolyzable py mimetic as the core structure, such as malonates, aryl carboxylates, phosphonates, or cinnamates. the poor membrane permeability of these inhibitors might compromise their potential development. it was reported that several α-bromoacetophenone derivatives, such as ptp inhibitor ii, could act as fairly potent ptp inhibitors, by covalently alkylating the conserved catalytic cysteine in the ptp active site. since ptp inhibitor ii is neutral, it could readily diffuse into human b cells and inhibit the intracellular ptps. the sar study was performed with the catalytic domain of phosphatase shp-1, and ti was found that ptp inhibitor ii showed time-dependent inactivation of shp-1, consistent with the mechanism. furthermore, the potency of ptp inhibitor ii was described by an equilibrium constant ki, representing the dissociation constant of the noncovalent enzyme–inhibitor complex. ptp inhibitor ii bound with lower affinity than ptp inhibitor i with ki values of 128 μm [1].
References
[1] p. heneberg. use of protein tyrosine phosphatase inhibitors as promising targeted therapeutic drugs. current medicinal chemistry 16(6), 706-733 (2009).
Properties of 2-Bromo-4'-methoxyacetophenone
| Melting point: | 69-71 °C(lit.) |
| Boiling point: | 215.8°C (rough estimate) |
| Density | 1.4921 (rough estimate) |
| refractive index | 1.5500 (estimate) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Crystals or Crystalline Powder |
| color | Off-white to light brown |
| Water Solubility | It is soluble in DMSO, water (partly miscible), most organic solvents, and methanol. |
| BRN | 743112 |
| InChI | InChI=1S/C9H9BrO2/c1-12-8-4-2-7(3-5-8)9(11)6-10/h2-5H,6H2,1H3 |
| CAS DataBase Reference | 2632-13-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Ethanone, 2-bromo-1-(4-methoxyphenyl)-(2632-13-5) |
Safety information for 2-Bromo-4'-methoxyacetophenone
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-Bromo-4'-methoxyacetophenone
| InChIKey | XQJAHBHCLXUGEP-UHFFFAOYSA-N |
| SMILES | C(=O)(C1=CC=C(OC)C=C1)CBr |
2-Bromo-4'-methoxyacetophenone manufacturer
JSK Chemicals
Swadev Chemicals
Rivashaa Agrotech Biopharma Pvt. Ltd.
Pratap Organics Pvt Ltd
Mahrshee Laboratories Pvt Ltd
Navone Specialties (OPC) Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2632-13-5 4'-METHOXYPHENACYL BROMIDE 98%View Details
2632-13-5 4'-METHOXYPHENACYL BROMIDE 98%View Details
2632-13-5 -
 4 – METHOXY PHENACYL BROMIDE 2632-13-5 98%View Details
4 – METHOXY PHENACYL BROMIDE 2632-13-5 98%View Details
2632-13-5 -
 4-Methoxyphenacyl bromide CAS 2632-13-5View Details
4-Methoxyphenacyl bromide CAS 2632-13-5View Details
2632-13-5 -
 2-Bromo-4′-methoxyacetophenone, 97% CAS 2632-13-5View Details
2-Bromo-4′-methoxyacetophenone, 97% CAS 2632-13-5View Details
2632-13-5 -
 4'-Methoxyphenacyl Bromide CAS 2632-13-5View Details
4'-Methoxyphenacyl Bromide CAS 2632-13-5View Details
2632-13-5 -
 2-Bromo-4'-methoxyacetophenone 98%View Details
2-Bromo-4'-methoxyacetophenone 98%View Details -
 BROMO-4-METHOXY ACETOPHENONE CAS 2632-13-5View Details
BROMO-4-METHOXY ACETOPHENONE CAS 2632-13-5View Details
2632-13-5 -
 2-Bromo-4′-methoxyacetophenone CAS 2632-13-5View Details
2-Bromo-4′-methoxyacetophenone CAS 2632-13-5View Details
2632-13-5