CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Liquid |
|---|---|
| Color/Form | COLORLESS OR WHITE NEEDLES; TEND TO YELLOW ON EXPOSURE TO LIGHT |
| Odor | POWERFUL WOODY-PHENOLIC, YET SOMEWHAT SWEET |
| Taste | SWEET SMOKEY |
| Boiling Point | 217.9 °C |
| Melting Point | 46 °C |
| Flash Point | 104 °C |
| Solubility | SLIGHTLY SOL IN WATER; SOL IN ALC, ETHER, & BENZENE; SOL IN CARBON DISULFIDE, & ACETONE |
| Density | 1.011 @ 20 °C |
| Vapor Pressure | 0.03 [mmHg] |
| LogP | Log Kow = 2.58 |
| Refractive Index | INDEX OF REFRACTION: 1.5239 @ 25 °C |
| Dissociation Constants | pK = 10.38 @ 20 °C |
| Kovats Retention Index | 1136 1145 1142 1162 1130 1136 1141 1137 1136 1133 1141 1170 1145 1147 1162 1137 1141 1141 1142 1162 |
| Other Experimental Properties | Liquid Heat of Formation = 0.123319 cu m/kmol |
| Chemical Classes | Other Classes -> Phenols |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 122.16 g/mol |
|---|---|
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 122.073164938 g/mol |
| Monoisotopic Mass | 122.073164938 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 72.6 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
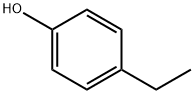
4-ethylphenol is a member of the class of phenols carrying an ethyl substituent at position 4. It has a role as a fungal xenobiotic metabolite.