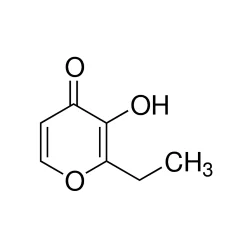
Ethyl maltol
Synonym(s):2-Ethyl-3-hydroxy-4H-pyran-4-one;Ethyl maltol
- CAS NO.:4940-11-8
- Empirical Formula: C7H8O3
- Molecular Weight: 140.14
- MDL number: MFCD00059795
- EINECS: 225-582-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 19:45:46
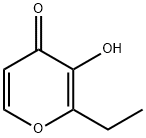
What is Ethyl maltol?
Chemical properties
White crystalline solid with characteristic, very sweet, caramel-like odor and taste. In dilute solution it possesses a sweet, fruitlike flavor and odor.
Chemical properties
It forms white crystals (mp 90–91°C) with very sweet caramel-like odor, four to six times more potent than maltol. Several syntheses have been developed for its preparation. In a one-pot process, for example, ??-ethylfurfuryl alcohol is treated with halogen to give 4-halo- 6-hydroxy-2-ethyl-2H-pyran-3(6H)-one, which need not be isolated and can be converted to ethylmaltol by aqueous hydrolysis
Chemical properties
Ethyl maltol has a very sweet, fruit-like odor of immense tenacity and sweet, fruity taste with initial bitter-tart flavor; rapid loss of flavor per se. It is four to six times more potent than maltol.
Occurrence
Has apparently not been reported to occur in nature.
History
In 1891, Bernhardi first discovered synthesis, and its derivatives are used for therapeutic purposes and can be prepared in industrial experiments. As war ammunition, it was first used by France in fortress warfare, and was prepared before the war. In October 1914, the French used it as a gas grenade, planned for the fortress battle. On January 7, 1915, it was actually used in the forest of Argonnen on the western front of France.
The Uses of Ethyl maltol
Ethyl Maltol is a flavoring agent that is a white, crystalline powder. it has a unique odor and a sweet taste that resembles fruit. the melt- ing point is 90°c. it is sparingly soluble in water and propylene gly- col and soluble in alcohol and chloroform. it is obtained by chemical synthesis.
The Uses of Ethyl maltol
Flavor and fragrance enhancer in foods, especially baked goods, beverages, and synthetic berry and citrus flavorings; minimizes undesirable flavors in tobacco products, cough syrup, vitamins, cosmetics, and saccharin-containing products.
The Uses of Ethyl maltol
Ethyl Maltol is an extract from medicinal plants such as P. Incarnata and can be used as an anticonvulsant, acting as a depressant, and on motor activity.
Production Methods
Unlike maltol, ethyl maltol does not occur naturally. It may be prepared by treating a-ethylfurfuryl alcohol with a halogen to produce 4-halo-6-hydroxy-2-ethyl-2H-pyran-3(6H)-one, which is converted to ethyl maltol by hydrolysis.
Preparation
Several syntheses have been developed for its preparation. In a one-pot process, for example, ??-ethylfurfuryl alcohol is treated with halogen to give 4-halo- 6-hydroxy-2-ethyl-2H-pyran-3(6H)-one, which need not be isolated and can be converted to ethylmaltol by aqueous hydrolysis.
Preparation
Fermentation method. Kojic acid is obtained from starch fermentation, and then ethyl maltol is obtained by etherification, oxidation, debenzylation, decarboxylation, hydroxylation and reduction.
Pyrofuroic acid method. A solution of pyrofuroic acid and acetic acid at a temperature of 90°C was added dropwise to the ether solution of diacetyl peroxide within 1~2h, and then the mixture was raised to 2h within 2h. 110 ° C, so that the 2-position of pyrofuroic acid can be directly alkylated to obtain ethyl maltol.
furfuryl alcohol method. Furfuryl alcohol is chlorinated in methanol aqueous solution by introducing chlorine gas to generate 4-chloro-3-hydroxy-4H-ketone, and then heated and hydrolyzed to obtain pyrofuroic acid; under alkaline conditions, pyrofuroic acid is condensed with acetaldehyde to obtain hydroxyethyl Pyrofuroic acid, which is reduced to ethyl maltol with zinc powder in hydrochloric acid.
Furfural method. Furfural reacts with ethylmagnesium bromide to obtain ethylfurfuryl alcohol (α-furan alkanol), which is then oxidized by chlorine gas in methanol aqueous solution at 0°C, and then heated to 100°C for hydrolysis to obtain ethyl maltol.
Definition
ChEBI: Ethyl maltol is a pyranone.
Taste threshold values
Taste characteristics at 70 ppm: sweet, burnt cotton, sugar candy-like with jamy, strawberry notes.
General Description
Ethyl maltol is a synthetic homologue of maltol, often found as flavor enhancers and it contributes to the fragrance of commercials products such as cereals, breads, malt beverages, coffee, soybeans and chocolate milk.
Flammability and Explosibility
Not classified
Pharmaceutical Applications
Ethyl maltol is used in pharmaceutical formulations and food products as a flavoring agent or flavor enhancer in applications similar to maltol. It has a flavor and odor 4–6 times as intense asmaltol. Ethyl maltol is used in oral syrups at concentrations of about 0.004% w/v and also at low levels in perfumery.
Safety Profile
Moderately toxic by ingestion and subcutaneous routes. Mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes.
Safety
In animal feeding studies, ethyl maltol has been shown to be well
tolerated with no adverse toxic, reproductive, or embryogenic effects. It has been reported that while the acute toxicity of ethyl
maltol, in animal studies, is slightly greater than maltol, with
repeated dosing the opposite is true. The WHO has set an
acceptable daily intake for ethyl maltol at up to 2 mg/kg bodyweight.
LD50 (chicken, oral): 1.27 g/kg
LD50 (rat, oral): 1.15 g/kg
LD50 (mouse, oral): 0.78 g/kg
LD50 (mouse, SC): 0.91 g/kg
Synthesis
From kojic acid
Metabolism
When orally administered, ethyl maltol was rapidly and extensively absorbed. Elimination was also extensive and rapid, involving conjugation as the glucuronide and ethereal sulphate, and excretion in the urine to the extent of 65-70% within 24 hr. Rate studies after iv dosage indicated that the bulk (86%) of the recovered conjugates was excreted within 6 hr (Rennhard, 1971).
Storage
Solutions may be stored in glass or plastic containers. The bulk material should be stored in a well-closed container, protected from light, in a cool, dry place.
Regulatory Status
GRAS listed. Included in the FDA Inactive Ingredients Database (oral syrup).
Properties of Ethyl maltol
| Melting point: | 85-95 °C (lit.) |
| Boiling point: | 196.62°C (rough estimate) |
| Density | 1.1624 (rough estimate) |
| vapor pressure | 0.2Pa at 24℃ |
| refractive index | 1.4850 (estimate) |
| FEMA | 3487 | ETHYL MALTOL |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | neat |
| pka | 8.38±0.10(Predicted) |
| form | Solid |
| color | White to Pale Yellow |
| Odor | at 5.00 % in benzyl alcohol. sweet caramel jam strawberry cotton candy |
| Water Solubility | 9.345g/L at 24℃ |
| JECFA Number | 1481 |
| Merck | 14,3824 |
| CAS DataBase Reference | 4940-11-8(CAS DataBase Reference) |
| NIST Chemistry Reference | 4H-pyran-4-one, 2-ethyl-3-hydroxy-(4940-11-8) |
| EPA Substance Registry System | 4H-Pyran-4-one, 2-ethyl-3-hydroxy- (4940-11-8) |
Safety information for Ethyl maltol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Ethyl maltol
Ethyl maltol manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 4940-11-08 0 Ethyl Maltol 99%View Details
4940-11-08 0 Ethyl Maltol 99%View Details
4940-11-08 0 -
 Ethyl maltol, ≥99% CAS 4940-11-8View Details
Ethyl maltol, ≥99% CAS 4940-11-8View Details
4940-11-8 -
 Ethyl maltol 98% CAS 4940-11-8View Details
Ethyl maltol 98% CAS 4940-11-8View Details
4940-11-8 -
 2-Ethyl-3-hydroxy-4-pyrone CAS 4940-11-8View Details
2-Ethyl-3-hydroxy-4-pyrone CAS 4940-11-8View Details
4940-11-8 -
 Ethyl Maltol CASView Details
Ethyl Maltol CASView Details -
 Ethyl Maltol, PowderView Details
Ethyl Maltol, PowderView Details
4940-11-8 -
 Ethyl Maltol, PowderView Details
Ethyl Maltol, PowderView Details
4940-11-8 -
 Ethyl MaltolView Details
Ethyl MaltolView Details
4940-11-8
