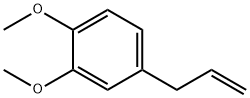
Estragole
Synonym(s):Estragole;Methyl chavicol;4-Allylanisole;Chavicol methyl ether;Isoanethole
- CAS NO.:140-67-0
- Empirical Formula: C10H12O
- Molecular Weight: 148.2
- MDL number: MFCD00008653
- EINECS: 205-427-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-20 21:15:22
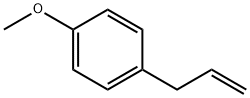
What is Estragole?
Chemical properties
colourless liquid with an aniseed smell
Chemical properties
Basil oil, methyl chavicol (estragole)-type (Réunion type, exotic type) is
obtained by steam distillation of the flowering tops or whole plants of
Ocimum basilicum L. (Lamiaceae), which was formerly grown in Réunion
and Madagascar. Today, the oil is produced in India and Vietnam on
relatively large scale (~100 and 30 t/yr, respectively) and also in Egypt, but
in small quantities. It is a light yellow liquid with a fresh, green, spicy odor
characteristic of methyl chavicol (estragole).
d2020 0.948–0.970; n20D 1.5100–1.5200; α20D ?1°to +2°; solubility: 1 vol in ≤7
vol 80% ethanol; content by GC: methyl chavicol 75–87%; linalool 0.5–3%(data for the classical “exotic” type; Indian types may contain up to
~20% linalool).
Chemical properties
Estragole has an odor reminiscent of anise with a corresponding sweet taste (differing from anethole)
Chemical properties
Herbaceous plant native to eastern and central Europe; it prefers shady growing sites. It grows 30 to 80 cm (12 to 31 in.) tall, with roots clustered in bundles, a branched stalk, alternate leaves and yellow flowers. Leaves are fragrant and anise-flavored. The plant flowers from July to August. The parts used are the flowering tops and leaves. Tarragon has a sweet, spicy odor and a sweet, anisic, fresh, green flavor reminiscent of basil and anise. This herb is commonly used to flavor vinegar and to season meats, soups, vegetable and fish dishes.
Occurrence
Reported found in anise oil.
The Uses of Estragole
4-Allylanisole has been used as a reference standard for the analysis of 4-allylanisole in essential oils by high performance liquid chromatography (HPLC) equipped with fluorometric detector and in emissions from live vegetation by solid-phase microextraction (SPME) combined with proton transfer reaction mass spectrometry (PTR-MS). It may be used as a reference standard for the determination of 4-allylanisole in food products by simultaneous distillation-extraction (SDE) followed by analyses using capillary gas chromatography (GC)-flame ionization detector (FID) and GC-MS.
The Uses of Estragole
insect atttractant, skin irritant, carcinogen
The Uses of Estragole
In perfumes and as flavor in foods and liqueurs.
What are the applications of Application
4-Allylanisole is a phenylpropene used in fragrances and the main component of basil oil
Preparation
Obtained by fractional distillation of the oil of turpentine or by treating a solution of the same oil in ether with an aqueous solution of mercuric acetate and subsequently heating the aqueous phase with zinc and sodium hydroxide; forms allyl bromide and magnesium p-methoxy phenate in ether.
Definition
ChEBI: Estragole is an olefinic compound.
Composition
The herb is reported to contain polyacetylenic compounds (capillene; phenyl-1,3-pentadiyne; acetylenic alcohol and its glucoside; capillone and dehydrofalcarinone); coumarinic and isocoumarinic derivatives (artemidinol, artemidiol, scopoletine, herciarine); alcohols (9-hydroxygeraniol; 4-methoxybenzyl alcohol); aldehyde (CoE, 2000).
Taste threshold values
Taste characteristics at 10 ppm: sweet, licorice, phenolic, weedy, spice, celery-like
Synthesis Reference(s)
The Journal of Organic Chemistry, 61, p. 1748, 1996 DOI: 10.1021/jo9518314
Synthesis, p. 701, 1983
General Description
Colorless liquid with odor of anise. Insoluble in water. Isolated from rind of persea gratissima grath. and from oil of estragon. Found in oils of Russian anise, basil, fennel turpentine, tarragon oil, anise bark oil.
Air & Water Reactions
Forms azeotropic mixtures with water. . Insoluble in water.
Reactivity Profile
4-Allylanisole may react vigorously with strong oxidizing agents. Can react exothermically with reducing agents (such as alkali metals and hydrides) to release gaseous hydrogen. May react exothermically with both acids and bases.
Health Hazard
ACUTE/CHRONIC HAZARDS: 4-Allylanisole is an irritant.
Fire Hazard
4-Allylanisole is combustible.
Safety Profile
Moderate acute toxicity by many routes. A skin irritant. Questionable carcinogen with experimental carcinogenic and neoplastigenic data. Mutation data reported. Combustible liquid. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALLYL COMPOUNDS. A spice used in foods, liqueurs, and perfumes.
Properties of Estragole
| Melting point: | 25°C |
| Boiling point: | 215-216 °C (lit.) |
| Density | 0.965 g/mL at 25 °C (lit.) |
| vapor pressure | 82-101hPa at 20-37.8℃ |
| FEMA | 2411 | ESTRAGOLE |
| refractive index | n |
| Flash point: | 178 °F |
| storage temp. | Sealed in dry,2-8°C |
| solubility | 0.178g/l |
| form | Liquid |
| color | Clear colorless |
| Specific Gravity | 0.965 |
| Odor | at 10.00 % in propylene glycol. sweet sassafrass anise spice green herbal fennel |
| Water Solubility | 177.8mg/L(25 ºC) |
| Merck | 14,3705 |
| JECFA Number | 1789 |
| BRN | 1099454 |
| Stability: | Stable. Flammable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 140-67-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzene, 1-methoxy-4-(2-propenyl)-(140-67-0) |
| EPA Substance Registry System | Estragole (140-67-0) |
Safety information for Estragole
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H341:Germ cell mutagenicity H351:Carcinogenicity H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Estragole
Estragole manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 Methyl Chavicol (Estragole) 98%View Details
Methyl Chavicol (Estragole) 98%View Details -
 4-Allylanisole 99%View Details
4-Allylanisole 99%View Details
140-67-0 -
 4-Allylanisole, 98% CAS 140-67-0View Details
4-Allylanisole, 98% CAS 140-67-0View Details
140-67-0 -
 4-Allylanisole CAS 140-67-0View Details
4-Allylanisole CAS 140-67-0View Details
140-67-0 -
 Estragole CAS 140-67-0View Details
Estragole CAS 140-67-0View Details
140-67-0 -
 4-Allylanisole CAS 140-67-0View Details
4-Allylanisole CAS 140-67-0View Details
140-67-0 -
 Estragole CAS 140-67-0View Details
Estragole CAS 140-67-0View Details
140-67-0 -
 Methyl Chavicol 98%, LiquidView Details
Methyl Chavicol 98%, LiquidView Details
140-67-0
