Allyl bromide
Synonym(s):3-Bromo-1-propene;Allyl bromide
- CAS NO.:106-95-6
- Empirical Formula: C3H5Br
- Molecular Weight: 120.98
- MDL number: MFCD00000244
- EINECS: 203-446-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13

What is Allyl bromide?
Chemical properties
Allyl bromide is a highly flammable, colourless to pale yellow transparent liquid possessing a strong, pungent, persistent odour. It is insoluble in water but soluble in alcohol, diethyl ether, acetone, carbon tetrachloride and chloroform.
The Uses of Allyl bromide
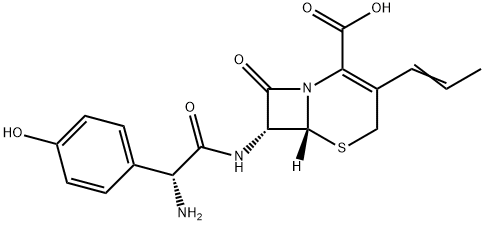
Allyl bromide is an alkylating agent commonly employed as an organic synthesis intermediate, finding extensive application in the preparation of pharmaceuticals, polymers, adhesives, fragrances, pesticides, and other allylic compounds. For instance: it reacts with zinc to produce precursors for allyl zinc bromide. It is also used in the preparation of allyl ether compounds, resveratrol derivatives, and the R-enantiomer of allylphenylcarbinol (APC) (1-phenyl-3-butenyl).
General Description
A clear colorless to light yellow liquid with an irritating unpleasant odor. Flash point 30°F. Irritates eyes, skin, and respiratory system. Toxic by skin absorption. Denser than water and slightly soluble in water.
Air & Water Reactions
Highly flammable. Slightly soluble in water.
Reactivity Profile
Allyl bromide decomposes upon heating and exposure to light, forming HBr (a strong reducing agent). Reacts violently with oxidizing agents. Can react exothermically with reducing agents to release hydrogen gas. In the presence of various catalysts (such as acids) or initiators, may undergo exothermic addition polymerization reactions.
Health Hazard
Exposures to allyl bromide cause severe eye and skin burns, irritation to the eyes, skin, and respiratory system. It is harmful when absorbed through the skin or inhaled in the workplace. Laboratory rats exposed for a prolonged period of time developed symptoms of poisoning, such as excessive salivation in a small number of animals, and severe gastric irritation. Vapors of allyl bromide may cause dizziness or suffocation, headache, coughing, and distressed breathing.
Flammability and Explosibility
Highly flammable
Safety Profile
Poison by ingestion and intraperitoneal routes. Mdly toxic by inhalation. Human mutation data reported. See also ALLYL CHLORIDE and ALLYL COMPOUNDS. Dangerous fire and explosion hazard when exposed to heat, flame, or oxidizers. When heated to decomposition it emits toxic fumes of Br-. To fight fire, use alcohol foam, water spray or mist, CO2, dry chemical
Synthesis
Allyl alcohol was synthesized from glycerol and formic acid under inert atmosphere, hydrolysed with NaOH and fractionally distilled to yield the 73% allyl alcohol water azeotrope. This was then reacted with 48% hydrobromic acid and sulfuric acid and the allyl bromide distilled as per the conventional method. It was then redistilled with 3A molecular sieves drying agent to yield the final product which is stored over additional 3A molecular sieves.
Potential Exposure
Used as an insecticide; in the manufacture of resins, fragrances, and other chemicals
Shipping
UN1099 Allyl bromide, Hazard Class: 3; Labels: 3-Flammable liquid, 6.1-Poisonous materials
Purification Methods
Wash the bromide with NaHCO3 solution then distilled water, dry (CaCl2 or MgSO4), and fractionally distil. Protect it from strong light. [Beilstein 1 IV 754.] LACHRYMATORY, HIGHLY TOXIC and FLAMMABLE.
Incompatibilities
Vapor may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Heat or light exposure may cause decomposition and corrosive vapors.
Waste Disposal
In accordance with 40CFR 165 recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Precautions
Workers should wear positive pressure self-contained breathing apparatus (SCBA), goggles and a face shield, protective clothing for high concentrations of vapor, chemical protective clothing that is specifi cally recommended by the manufacturer to avoid poisoning. Workers should be careful as allyl bromide reacts with oxidizing materials and alkalis.
Properties of Allyl bromide
| Melting point: | -119 °C |
| Boiling point: | 70-71 °C(lit.) |
| Density | 1.398 g/mL at 25 °C(lit.) |
| vapor density | 4.2 (vs air) |
| vapor pressure | 18.66kPa at 25℃ |
| refractive index | n |
| Flash point: | 28 °F |
| storage temp. | 2-8°C |
| solubility | Chloroform (Soluble) |
| form | Liquid |
| color | Clear colorless to slightly colored |
| Specific Gravity | 1.398 |
| Odor | unpleasant |
| explosive limit | 4.3-7.3%(V) |
| Water Solubility | insoluble |
| Sensitive | Light Sensitive |
| Merck | 14,288 |
| BRN | 605308 |
| Exposure limits | ACGIH: TWA 0.1 ppm; STEL 0.2 ppm (Skin) |
| Dielectric constant | 7.0 |
| Stability: | Stable. Flammable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 106-95-6(CAS DataBase Reference) |
| NIST Chemistry Reference | 1-Propene, 3-bromo-(106-95-6) |
| EPA Substance Registry System | 3-Bromopropene (106-95-6) |
Safety information for Allyl bromide
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H225:Flammable liquids H314:Skin corrosion/irritation H340:Germ cell mutagenicity H350:Carcinogenicity H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Allyl bromide
| InChIKey | BHELZAPQIKSEDF-UHFFFAOYSA-N |
Allyl bromide manufacturer
JSK Chemicals
Sigma Inc
Jaiswal S Cyber Shop
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


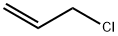
You may like
-
 Allyl bromide 99%View Details
Allyl bromide 99%View Details -
 Allyl bromide, Stabilized CAS 106-95-6View Details
Allyl bromide, Stabilized CAS 106-95-6View Details
106-95-6 -
 Allyl Bromide pure CAS 106-95-6View Details
Allyl Bromide pure CAS 106-95-6View Details
106-95-6 -
 Allyl BromideView Details
Allyl BromideView Details
106-95-6 -
 Liquid JCSSUPER 106-95-6 Allyl bromide for synthesis 250 ml., Grade Standard: Reagent GradeView Details
Liquid JCSSUPER 106-95-6 Allyl bromide for synthesis 250 ml., Grade Standard: Reagent GradeView Details
106-95-6 -
 Reagent Grade Allyl BromideView Details
Reagent Grade Allyl BromideView Details
106-95-6 -
 Sigma Inc Allyl Bromide Chemical, For Polymers,Pharmaceuticals, Grade Standard: Analytical GradeView Details
Sigma Inc Allyl Bromide Chemical, For Polymers,Pharmaceuticals, Grade Standard: Analytical GradeView Details
106-95-6 -
 Allyl Bromide ChemicalView Details
Allyl Bromide ChemicalView Details
106-95-6
