Ko 143
Synonym(s):(3S,6S,12aS)-1,2,3,4,6,7,12,12a-Octahydro-9-methoxy-6-( 2-methylpropyl)-1,4-dioxopyrazino[1′,2′:1,6]pyrido[3,4- b]indole-3-propanoic acid 1,1-dimethylethyl ester hydrate;Ko-143 hydrate
- CAS NO.:461054-93-3
- Empirical Formula: C26H35N3O5
- Molecular Weight: 469.57
- MDL number: MFCD11042273
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
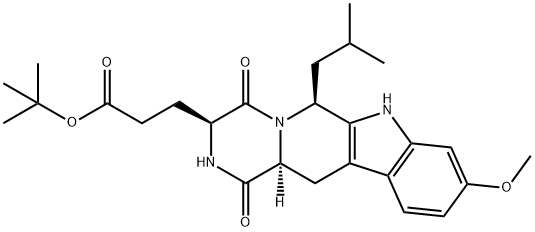
What is Ko 143?
Description
Breast cancer resistance protein (BCRP) is an ATP-
The Uses of Ko 143
A potent BCRP (breast cancer resistance protein) inhibitor
The Uses of Ko 143
Ko143 hydrate has been used:
- to determine the role of ATP-binding cassette sub-family G member 2 (ABCG2), human embryonic kidney (HEK)-C1 and HEK-ABCG2 in tumor microenvironment
- to inhibit ABCG2 for sphere formation assay
- in calcein-AM efflux inhibition to monitor multidrug resistance protein (MRP)-function in kidney
- for cell viability assay
What are the applications of Application
Ko 143 is a selective BCRP multidrug transporter inhibitor
Definition
ChEBI: LSM-6260 is a member of beta-carbolines and a tert-butyl ester.
Biological Activity
Potent and selective breast cancer resistance protein multidrug transporter (BCRP) inhibitor (EC 90 = 26 nM). Displays > 200-fold selectivity over P-gp and MRP-1 transporters. Increases intracellular drug accumulation and reverses BCRP-mediated multidrug resistance.
Biochem/physiol Actions
Ko143 has been used as a positive control inhibitor on functions of breast cancer resistance protein (BCRP) using a BCRP prototypical substrate mitoxantrone. BCRP, an ABCG2 transporter, plays an important role in disposition of many drugs and environmental toxins. Ko143 displays > 200-fold selectivity over P-gp and MRP-1 transporters and thus is more specific than other known BCRP inhibitors such as fumitremorgin C and GF120918. It increases intracellular drug accumulation and reverses BCRP-mediated multidrug resistance. It blocks topotecan and ABZSO transport in a concentration-dependent manner.
storage
Store at -20°C
References
1) Allen et al. (2002), Potent and specific inhibition of breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C; Mol. Cancer Ther., 1 417 2) Weidner et al. (2015), The Inhibitor Ko143 Is Not Specific for ABCG2; J. Pharmacol. Exp. Ther., 354 384 3) Palasuberniam et al. (2015), ABCG2 transporter inhibitor restores the sensitivity of triple negative breast cancer cells to aminolevulinic acid-mediated photodynamic therapy; Sci. Rep., 5 13298 4) Moreno-Sanz et al. (2011), The ABC membrane transporter ABCG2 prevents access of FAAH inhibitor URB937 to the central nervous system; Pharmacol. Res., 64 359 5) Sabnis et al. (2017),?The Efflux Transporter ABCG2 Maintains Prostate Stem Cells;? Mol. Cancer Res., 15 128
Properties of Ko 143
| Melting point: | 147 ºC |
| Boiling point: | 689.8±55.0 °C(Predicted) |
| Density | 1.24 |
| storage temp. | Desiccate at -20°C |
| solubility | DMSO: >10mg/mL |
| form | powder |
| pka | 13.31±0.60(Predicted) |
| color | white to off-white |
| Stability: | Stable for 2 years from date of purchase as supplied. Protect from exposure to moisture. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. |
Safety information for Ko 143
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ko 143
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 Ko143 hydrate CAS 461054-93-3View Details
Ko143 hydrate CAS 461054-93-3View Details
461054-93-3 -
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4