3,7-Dimethyl-7-hydroxyoctanal
Synonym(s):7-Hydroxy-3,7-dimethyloctanal;Citronellal hydrate
- CAS NO.:107-75-5
- Empirical Formula: C10H20O2
- Molecular Weight: 172.26
- MDL number: MFCD00014681
- EINECS: 203-518-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:53
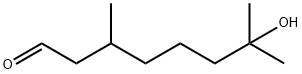
What is 3,7-Dimethyl-7-hydroxyoctanal?
Description
Hydroxycitronellal has an intense, sweet, floral, lily-type odor. It may be prepared by hydration of natural citronellal obtained from Java citronella or from Eucalyptus citriodora; P-pinene is converted to myrcene, which on hydration may yield either linalool or a mixture of geranoil and nerol; the latter mixture can be hydrogenated to citronellol and subsequently converted to citronellal and hydroxycitronellal; also by hydrogenation of 3,7-dimethyl- 7-hydroxy-2-octen-2-al over palladium carbon in ethyl acetate solution.
Chemical properties
clear colourless liquid
Chemical properties
This is a colorless,
slightly viscous liquid with a floral odor reminiscent of linden blossom and lily of
the valley. Commercially available “hydroxycitronellal” is either optically active
or racemic, depending on the starting material used. Hydroxydihydrocitronellal
prepared from (+)-citronellal, for example, has a specific relation α20
D +9 to +10°.
Hydroxydihydrocitronellal is relatively unstable toward acid and alkali and is,
therefore, sometimes converted into more alkali-resistant acetals, particularly its
dimethyl acetal.
Because of its fine, floral odor, hydroxydihydrocitronellal is used in large
quantities in many perfume compositions for creating linden blossom and lily of
the valley notes. It is also used in other blossom fragrances such as honeysuckle,
lily, and cyclamen.
Chemical properties
Hydroxycitronellal has a sweet, floral, lily-type odor
Occurrence
Reported found in pepper
The Uses of 3,7-Dimethyl-7-hydroxyoctanal
Hydroxycitronellal is a fragrance for use in various perfumes, antiseptics, insecticides and household products; some perfumery uses (sweet pea Pois De Senteur; gardenia; cherry; melon; mint).
The Uses of 3,7-Dimethyl-7-hydroxyoctanal
7-Hydroxycitronellal shows fungicide activity the strains of Candida tropicalis.
Background
Hydroxycitronellal is a synthetic fragrance that is widely used in many cosmetics and hygiene products such as deodorants, soaps, antiseptics, and other household items. It has the smell of lilac, lily, and lily of the valley . Hydroxycitronellal has also been shown to be a dermatologic irritant and allergen, and as a result commercially available products are restricted by the International Fragrance Association (IFRA) to contain only 0.1-3.6%.
Sensitivity to hydroxycitronellal may be identified with a clinical patch test.
Indications
Hydroxycitronellal is approved by the FDA for use within allergenic epicutaneous patch tests which are indicated for use as an aid in the diagnosis of allergic contact dermatitis (ACD) in persons 6 years of age and older.
Definition
ChEBI: The tertiary alcohol arising from addition of water across the C2C double bond of citronellal.
Preparation
The most important synthetic routes to hydroxydihydrocitronellal
are listed as follows.
1) Synthesis from citronellal: One of the oldest routes to hydroxydihydrocitronellal
is the hydration of the citronellal bisulfite adduct (obtained at low
temperature) with sulfuric acid, followed by decomposition with sodium
carbonate. A more recent development is hydration of citronellal enamines
or imines, followed by hydrolysis.
2) Synthesis from citronellol. Citronellol is hydrated to 3,7-dimethyloctane-1,7-
diol, for example, by reaction with 60% sulfuric acid. The diol is dehydrogenated
catalytically in the vapor phase at low pressure to give highly pure
hydroxydihydrocitronellal in excellent yield.The process is carried out in the
presence of, for example, a copper–zinc catalyst; at atmospheric pressure,
noble metal catalysts can also be used.
3) Synthesis from 7-hydroxygeranyl/-neryl dialkylamine: The starting material
can be obtained by treatment of myrcene with a dialkylamine in the
presence of an alkali dialkylamide, followed by hydration with sulfuric
acid. The 7-hydroxygeranyl/-neryl dialkylamine isomerizes to the corresponding
7-hydroxyaldehyde enamine in the presence of a palladium(II)-
phosphine complex as catalyst. Hydrolysis of the enamine gives 7-hydroxydihydrocitronellal
[151].
Taste threshold values
Taste characteristics at 50 ppm: sweet, waxy, green, floral and melon notes.
Flammability and Explosibility
Not classified
Trade name
Laurinal® (Takasago).
Contact allergens
Hydroxycitronellal is a classical fragrance allergen, found in many products. It is contained in “fragrance mix.” It has to be listed by name in the cosmetics of the EU.
Safety Profile
A skin irritant. Combustible liquid. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALDEHYDES.
Synthesis
By hydration of natural citronellal obtained from Java citronella or from Eucalyptus citriodora; β-pinene is converted to myrcene, which on hydration may yield either linalool or mixture of geranoil and nerol; the latter mixture can be hydrogenated to citronellol and subsequently converted to citronellal and hydroxycitronellal; also by hydrogenation of 3,7-dimethyl-7-hydroxy-2- octen-2-al over palladium carbon in ethyl acetate solution.
Metabolism
Studies in rabbits have shown that hydroxycitronellal is converted to two primary metabolites: reduction to the alcohol 7-hydroxycitronellol and oxidation to the carboxylic acid 7-hydroxycitronellylic acid . On average, about 50% of hydroxycitronellal is excreted as 7-hydroxycitronellylic acid which is therefore regarded as the major metabolite .
Properties of 3,7-Dimethyl-7-hydroxyoctanal
| Melting point: | 22-23 °C |
| Boiling point: | 257 °C(lit.) |
| Density | 0.923 g/mL at 25 °C(lit.) |
| vapor pressure | 0.547Pa at 20℃ |
| refractive index | n |
| FEMA | 2583 | HYDROXYCITRONELLAL |
| Flash point: | >230 °F |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | Benzene (Slightly), Chloroform (Slightly), Methanol (Slightly) |
| pka | 15.31±0.29(Predicted) |
| form | Liquid |
| color | Clear colorless |
| Specific Gravity | 0.93 |
| Odor | at 100.00 %. floral lily sweet green waxy tropical melon |
| Water Solubility | 35g/L at 20℃ |
| JECFA Number | 611 |
| Stability: | Air Sensitive |
| CAS DataBase Reference | 107-75-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Hydroxycitronellal(107-75-5) |
| EPA Substance Registry System | Octanal, 7-hydroxy-3,7-dimethyl- (107-75-5) |
Safety information for 3,7-Dimethyl-7-hydroxyoctanal
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 3,7-Dimethyl-7-hydroxyoctanal
| InChIKey | WPFVBOQKRVRMJB-UHFFFAOYSA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran





You may like
-
 107-75-5 Hydroxy Citronellal 98%View Details
107-75-5 Hydroxy Citronellal 98%View Details
107-75-5 -
 Hydroxy citronellal CAS 107-75-5View Details
Hydroxy citronellal CAS 107-75-5View Details
107-75-5 -
 7-Hydroxycitronellal CAS 107-75-5View Details
7-Hydroxycitronellal CAS 107-75-5View Details
107-75-5 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
