3,4-Dinitrochlorobenzene
- CAS NO.:610-40-2
- Empirical Formula: C6H3ClN2O4
- Molecular Weight: 202.55
- MDL number: MFCD00007212
- EINECS: 210-223-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
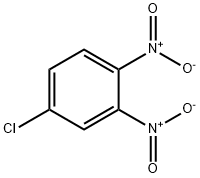
What is 3,4-Dinitrochlorobenzene?
Description
Securinine is derived from the leaf of Securinega suffruticosa Rehd., which was first separated by V.I.?Murav’eva in 1956 . Securinega suffruticosa Rehd., a kind of subshrub plant, is widely distributed in temperate and subtropical regions . It has strong adaptability and can be planted in most parts of China. Moreover, it is rich in natural resources. Securinega suffruticosa Rehd. is a folk medicine whose root is the main component, with the effects of activating circulation, spleen, and stomach to treat rheumatism waist pain, limb numbness, infant malnutrition, and other diseases. The roots, stems, leaves, flowers, and skin of Securinega suffruticosa Rehd. contain alkaloids, in which securinine is the main ingredient.
Chemical properties
YELLOW-BROWN OILY LIQUID AFTER MELTING
Physical properties
Appearance: yellow crystalline powder, slightly bitter. Solubility: soluble in anhydrous ethanol or chloroform, insoluble in water. Melting point: 139–140?°C Specific optical rotation: (D) ?1042°(C?=?1, EtOH).
History
Securinine was first isolated by a Soviet scholar from the Wusuli region, but its
chemical structure was separated and finally determined by Chinese scholars
from local sources . The main structural feature is a tetracyclic compound containing an indolizine, pyrrole elidine, or quinolizine ring and an α, β-unsaturated
pentahydrin ring whose basic structural skeleton type is as shown in what follows .
Owing to the complexity of the chemical reaction of securinine, reports on its
chemical reactivity and structural modification have been relatively scarce since
securinine was reported on in 1956. However, the research on chemical total synthesis and biological activities of securinine has made a certain amount of progress.
Securinine has a rigid molecular structure containing four rings and three chiral
centers, which makes synthesis difficult. From 1974 to 1978, three research groups
from Japan, the USA, and Canada studied the biosynthesis of securinine by feeding
animals with isotope-labeled S. suffruticosa.
The Uses of 3,4-Dinitrochlorobenzene
3,4-Dinitrochlorobenzene is a useful reactant in organic synthesis.
The Uses of 3,4-Dinitrochlorobenzene
This compound is a sensitizer.
Indications
Poliomyelitis sequela and facial paralysis, neurasthenia, hypotension, autonomic dysfunction, and others
Pharmacology
The pharmacological effect of securinine is mainly manifested as a central nervous
system excitatory effect. As a GABA receptor inhibitor, it has an excitatory effect
similar to that of strychnine on the spinal cord. A low dose of securinine can improve
the excitability of brain reflection, while a high dose of securinine will cause febrile
seizures, and at the same time securinine can strengthen the conditioned reflex of
the cerebral cortex, shorten the latency period, and promote learning and memory
capability , and so it is expected to be a promising drug for the treatment of
Alzheimer’s disease.,
Securinine can improve the hematopoietic environment of patients with aplastic anemia, promote cell proliferation, have a synergistic anti-tumor effect in combination with cyclophosphamide (CTX), and can antagonize bone marrow
suppression caused by CTX.?It has an inhibitory effect on cell proliferation of
human leukocyte cell K562 and four other kinds of tumor cells.
Clinical Use
In recent years, securinine has been widely used in clinical practice, mainly for the treatment of polio sequelae and facial nerve palsy. It also has a certain effect on neurasthenia, hypotension and dizziness, tinnitus, and deafness caused by autonomic dysfunction. The nitrate and hydrochloride of left-handed securinine are mainly used clinically. In addition, securinine eye drops are found to have a remarkable effect on the treatment of herpes simplex keratitis through initial clinical observation.
Properties of 3,4-Dinitrochlorobenzene
| Melting point: | 40-41 °C |
| Boiling point: | 16 °C (4 mmHg) |
| Density | 1.687 g/mL at 25 °C(lit.) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | 0-10°C |
| form | Oily Liquid After Melting |
| color | Yellow-brown |
| Water Solubility | insoluble |
| BRN | 521564 |
| CAS DataBase Reference | 610-40-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzene, 4-chloro-1,2-dinitro-(610-40-2) |
| EPA Substance Registry System | Benzene, 4-chloro-1,2-dinitro- (610-40-2) |
Safety information for 3,4-Dinitrochlorobenzene
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H310:Acute toxicity,dermal H317:Sensitisation, Skin H330:Acute toxicity,inhalation H371:Specific target organ toxicity, single exposure H373:Specific target organ toxicity, repeated exposure H400:Hazardous to the aquatic environment, acute hazard H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P272:Contaminated work clothing should not be allowed out of the workplace. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P320:Specific treatment is urgent (see … on this label). P330:Rinse mouth. P391:Collect spillage. Hazardous to the aquatic environment P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P501:Dispose of contents/container to..… |
Computed Descriptors for 3,4-Dinitrochlorobenzene
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 1-Chloro-3,4-dinitrobenzene CAS 610-40-2View Details
1-Chloro-3,4-dinitrobenzene CAS 610-40-2View Details
610-40-2 -
 3,4-Dinitrochlorobenzene CAS 610-40-2View Details
3,4-Dinitrochlorobenzene CAS 610-40-2View Details
610-40-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1