2,4-Dinitrochlorobenzene
Synonym(s):1-Chloro-2,4-dinitrobenzene;2,4-Dinitrochlorobenzene;4-Chloro-1,3-dinitrobenzene, 1,3-Dinitro-4-chlorobenzene, 2,4-Dinitro-1-chlorobenzene;CDNB;DNCB
- CAS NO.:97-00-7
- Empirical Formula: C6H3ClN2O4
- Molecular Weight: 202.55
- MDL number: MFCD00007075
- EINECS: 202-551-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
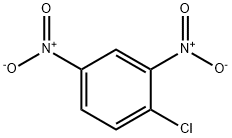
What is 2,4-Dinitrochlorobenzene?
Chemical properties
yellow crystals with an almond odour
The Uses of 2,4-Dinitrochlorobenzene
1-Chloro-2,4-dinitrobenzene is a benzene derivative and is used in biochemical research as a substrate for glutathione S-transferase.
The Uses of 2,4-Dinitrochlorobenzene
1-Chloro-2,4-dinitrobenzene is used as a reagent for the detection and determination of pyridine compounds. It has been used as alkylating agent to evaluate the depletion of intracellular erythrocyte glutathione (GSH). It is an irreversible inhibitor of human thioredoxin reductase.
What are the applications of Application
1-Chloro-2,4-dinitrobenzene is a reagent for the detection and determination of pyridine compounds
Definition
ChEBI: 2,4-Dinitrochlorobenzene is a C-nitro compound that is chlorobenzene carrying a nitro substituent at each of the 2- and 4-positions. It has a role as an epitope, an allergen and a sensitiser. It is a C-nitro compound and a member of monochlorobenzenes.
Production Methods
Although chlorobenzene can be dinitrated directly, this results in unnecessary isomer problems. 4-Chloronitrobenzene is usually nitrated with mixed acid (35/65) at 60 ℃ to give the pure dinitro isomer. However, 2- chloronitrobenzene can be nitrated to produce 2,4-Dinitrochlorobenzene, together with ca. 10 wt % of the isomeric 2- chloro- 1,3-dinitrobenzene. This may be separated for disposal, but the mixed isomers are preferably used directly if tolerated by the end product (e.g., sulfur dyes).
General Description
Pale yellow needles, almond odor.
Reactivity Profile
Self-reactive, [Halpern, Chem. and Eng. News, 29:2666(1951)]. The mixture of 2,4-Dinitrochlorobenzene with hydrazine hydrate caused a violent reaction.
Hazard
Toxic by ingestion, inhalation, and skin absorption. Combustible. Upper explosive limit 22%. A skin irritant.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
Contact allergens
This substance is one of the strongest primary skin irritants known, and a universal contact allergen. Occupational dermatitis has been reported, but current use is decreasing or performed with completely closed systems. DNCB is sometimes used for topical treatment of alopecia areata, severe warts, and cutaneous metastasis of malignant melanoma
Safety Profile
Poison by skin contact and intraperitoneal routes. Moderately toxic by ingestion. A severe human skin and eye irritant. Acts as a primary irritant as well as a sensitizer of skin. An allergen. Mutation data reported. Combustible when exposed to heat or flame. A moderate explosion hazard when exposed to flame, sparks, heated to 1 50°, or when shocked in a sealed container. Explosive reaction with ammonia at 17O℃/40 bar. To fight fire, use CO2, dry chemical. Reacts violently with hydrazine sulfate or hydrazine hydrate. See also NITRO COMPOUNDS of AROMATIC HYDROCARBONS.
Purification Methods
Usually it is recrystallised from EtOH or MeOH. It has also been crystallised from Et2O, *C6H6, *C6H6/pet ether or isopropyl alcohol. A preliminary purification step is to pass its solution in *benzene through an alumina column. It has also been purified by zone refining. It exists in three forms: one stable and two unstable. The stable form crystallises as yellow needles from Et2O, m 51o, b 315o/760mm with some decomposition, and is soluble in EtOH. A labile form also crystallises from Et2O, m 43o, and is more soluble in organic solvents. The second labile form has m 27o. [Hoffman & Dame, J Am Chem Soc 41 1015 1919, Welsh J Am Chem Soc 63 3276 1941, J Chem Soc 2476 1957, Beilstein 5 IV 744.]
Properties of 2,4-Dinitrochlorobenzene
| Melting point: | 48-50 °C(lit.) |
| Boiling point: | 315 °C(lit.) |
| Density | 314 |
| vapor density | 6.98 (vs air) |
| vapor pressure | 1 hPa (106 °C) |
| refractive index | 1.5857 |
| Flash point: | 367 °F |
| storage temp. | Store below +30°C. |
| solubility | alcohol: very slightly soluble (cold)(lit.) |
| form | Crystalline Mass, Chunks or Crystals and Powder |
| color | Yellow to brown |
| Odor | Almond-like |
| explosive limit | 1.9-22%(V) |
| Water Solubility | insoluble |
| Merck | 14,2136 |
| BRN | 613161 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, ammonia. Reacts violently with hydrazine hydrate. |
| CAS DataBase Reference | 97-00-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzene, 1-chloro-2,4-dinitro-(97-00-7) |
| EPA Substance Registry System | 2,4-Dinitrochlorobenzene (97-00-7) |
Safety information for 2,4-Dinitrochlorobenzene
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H310:Acute toxicity,dermal H315:Skin corrosion/irritation H317:Sensitisation, Skin H318:Serious eye damage/eye irritation H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P262:Do not get in eyes, on skin, or on clothing. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2,4-Dinitrochlorobenzene
2,4-Dinitrochlorobenzene manufacturer
Hemani Global
Colour Finders
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

You may like
-
 1-Chloro-2,4-Dinitrobenzene extrapure AR CAS 97-00-7View Details
1-Chloro-2,4-Dinitrobenzene extrapure AR CAS 97-00-7View Details
97-00-7 -
 1-Chloro-2,4-Dinitrobenzene extrapure CAS 97-00-7View Details
1-Chloro-2,4-Dinitrobenzene extrapure CAS 97-00-7View Details
97-00-7 -
 1-Chloro-2,4-dinitrobenzene CAS 97-00-7View Details
1-Chloro-2,4-dinitrobenzene CAS 97-00-7View Details
97-00-7 -
 1-Chloro-2,4-dinitrobenzene, GR CAS 97-00-7View Details
1-Chloro-2,4-dinitrobenzene, GR CAS 97-00-7View Details
97-00-7 -
 1-CHLORO-2,4-DINITROBENZENE For Synthesis CAS 97-00-7View Details
1-CHLORO-2,4-DINITROBENZENE For Synthesis CAS 97-00-7View Details
97-00-7 -
 1-CHLORO-2,4-DINITROBENZENE AR CAS 97-00-7View Details
1-CHLORO-2,4-DINITROBENZENE AR CAS 97-00-7View Details
97-00-7 -
 1-Chloro-2,4-dinitrobenzene CAS 97-00-7View Details
1-Chloro-2,4-dinitrobenzene CAS 97-00-7View Details
97-00-7 -
 2-4-DI NITRO CHLORO BENZENEView Details
2-4-DI NITRO CHLORO BENZENEView Details
97-00-7
