1,2,4-Trichlorobenzene
Synonym(s):1,2,4-TCB;1,2,4-Trichlorobenzene;acs solvents;high purity solvents
- CAS NO.:120-82-1
- Empirical Formula: C6H3Cl3
- Molecular Weight: 181.45
- MDL number: MFCD00000547
- EINECS: 204-428-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
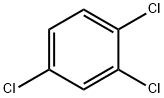
What is 1,2,4-Trichlorobenzene?
Description
Trichlorobenzenes (TCBs) are synthetic chemicals that occur in three different isomeric forms. The three chlorinated cyclic aromatic isomers are 1,2,3-trichlorobenzene (1,2,3-TCB), 1,2,4-trichlorobenzene (1,2,4-TCB), and 1,3,5-trichlorobenzene (1,3,5-TCB). 1,2,4-TCB is one of the 188 chemicals designated as a hazardous air pollutant under the Clean Air Act.
Chemical properties
1,2,4-Trichlorobenzene is a low-melting solid or liquid with a pleasant, aromatic odor. The Odor Threshold is 1.4 ppm.
Physical properties
Colorless liquid with an odor similar to o-dichlorobenzene. Odor threshold concentration is 1.4 (quoted, Amoore and Hautala, 1983). Miscible with most organic solvents and oils; insoluble in water. Combustible.
The Uses of 1,2,4-Trichlorobenzene
1,2,4-Trichlorobenzene is used as a dielectric and heat transfer fluid in transformers. It acts as an intermediate, degreaser, wood preservative and solvent for dye. It is a high-temperature solvent used in gel permeation chromatography, especially for polyethylene and polypropylene. Further, it is used as a lubricant and as a synthetic transformer oil.
What are the applications of Application
1,2,4-Trichlorobenzene is a compound used in soil and ground water contamination studies.
Definition
ChEBI: 1,2,4-trichlorobenzene is a trichlorobenzene with chloro substituents at positions 1, 2 and 4. It is a solvent in various organic chemical reactions.
What are the applications of Application
Trichlorobenzenes are primarily used as solvents in chemical manufacturing industries. 1,2,4-Trichlorobenzene is economically the most important isomer. It is used as a solvent in chemical reactions to dissolve oils, waxes, and resins. Furthermore, it is also used as a dye carrier. 1,2,4-Trichlorobenzene is a highly chlorinated aromatic solvent. It may be used as a solvent to prepare:
dimethylketene β-lactone dimer from tetramethyl-1,3-cyclobutanedione
salicyl-o-toluide by the reaction of phenyl salicylate and o-toluidine
Synthesis Reference(s)
Journal of the American Chemical Society, 74, p. 3890, 1952 DOI: 10.1021/ja01135a052
General Description
Colorless liquid or white solid with a sharp chlorobenzene odor. Melting point 16.95°C (62.5°F) .
Reactivity Profile
1,2,4-Trichlorobenzene can react vigorously with oxidizing materials . Yields hydrogen chloride and phosgene when heated to decomposition [USCG, 1999].
Health Hazard
Exposures to high concentrations via inhalation are potentially hazardous to the lungs, kidneys and liver. Prolonged or repeated exposures or short exposure to high concentrations via inhalation are potentially hazardous to the lungs, kidneys and liver. Prolonged or repeated exposure to the eyes is likely to result in moderate pain and transient irritation. Prolonged or repeated contact with the skin may result in moderate irritation and possible systemic effects. Ingestion: May cause kidney and liver damage.
Safety Profile
Poison by ingestion. Moderately toxic by intraperitoneal route. An experimental teratogen. Experimental reproductive effects. Mutation data reported. A slan irritant. Combustible when exposed to heat or flame. Can react vigorously with oxidizing materials. To fight fire, use water, foam, CO2, dry chemical. When heated to decomposition it emits toxic fumes of Cl-. See also CHLORINATED HYDROCARBONS, AROMATIC.
Potential Exposure
1,2,4-Trichlorobenzene is used as a dye carrier, herbicide intermediate; a heat transfer medium; a dielectric fluid in transformers; a degreaser; a lubricant; as an industrial chemical; solvent, emulsifier, and as a potential insecticide against termites. The other trichlorobenzene isomers are not used in any quantity.
Environmental Fate
Biological. Under aerobic conditions, biodegradation products may include 1,2-dichlorobenzene,
1,3-dichlorobenzene, 1,4-dichlorobenzene, and carbon dioxide (Kobayashi and
Rittman, 1982). A mixed culture of soil bacteria or a Pseudomonas sp. transformed 1,2,4-trichlorobenzene
to 2,4,5- and 2,4,6-trichlorophenol (Ballschiter and Scholz, 1980). When 1,2,4-
trichlorobenzene was statically incubated in the dark at 25 °C with yeast extract and settled
domestic wastewater inoculum, significant biodegradation occurred, with gradual acclimation
followed by a deadaptive process in subsequent subcultures. At a concentration of 5 mg/L, 54, 70,
59, and 24% losses were observed after 7, 14, 21, and 28-d incubation periods, respectively. At a
concentration of 10 mg/L, only 43, 54, 14, and 0% were observed after 7, 14, 21, and 28-d
incubation periods, respectively (Tabak et al., 1981). In activated sludge, <0.1% mineralized to
carbon dioxide after 5 d (Freitag et al., 1985).
In an enrichment culture derived from a contaminated site in Bayou d’Inde, LA, 1,2,4-
trichlorobenzene underwent reductive dechlorination to 1,3- and 1,4-dichlorobenzene at relative
molar yields of 4 and 96%, respectively. The maximum dechlorination rate, based on the
recommended Michaelis-Menten model, was 4.6 nM/d (Pavlostathis and Prytula, 2000).
Surface Water. Estimated half-lives of 1,2,4-trichlorobenzene (0.5 μg/L) from an experimental
marine mesocosm during the spring (8–16 °C), summer (20–22 °C), and winter (3–7 °C) were 22,
11, and 12 d, respectively (Wakeham et al., 1983).
Photolytic. A carbon dioxide yield of 9.8% was achieved when 1,2,4-trichlorobenzene adsorbed
on silica gel was irradiated with light (λ >290 nm) for 17 h (Freitag et al., 1985).
Chemical/Physical. The hydrolysis half-life was estimated to be >900 yr (Ellington et al., 1988).
At 70.0 °C and pH values of 3.10, 7.11, and 9.77, the hydrolysis half-lives were calculated to be
18.4, 6.6, and 5.9 d, respectively (Ellington et al., 1986).
At influent concentrations of 1.0, 0.1, 0.01, and 0.001 mg/L, the GAC adsorption capacities
were 157, 77.6, 38.4, and 19.0 mg/g, respectively (Dobbs and Cohen, 1980).
Shipping
UN2321 Trichlorobenzenes, liquid, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Separate it from a mixture of isomers by washing with fuming H2SO4, then water, drying with CaSO4 and slowly fractionally distilling. [Jensen et al. J Am Chem Soc 81 3303 1959, Beilstein 5 IV 664.]
Toxicity evaluation
The liver is themain target of trichlorobenzenes irrespective of the route of exposure. The mechanisms of liver toxicity induced by these chemicals have not been illustrated. It might involve intermediate arene oxides formed during initial transformation to trichlorophenols. In addition, exposure to 1,2,4-TCB induced porphyria in rats by inducing daminolevulinic acid (ALA) synthetase, a rate-limiting enzyme in the biosynthesis of heme, and also heme oxygenase, a ratelimiting enzyme in the degradation of heme synthetase, and therefore increasing heme production.
Incompatibilities
Reacts violently with oxidants, acids, acid fumes; steam.
Waste Disposal
Incineration, preferably after mixing with another combustible fuel. Care must be exercised to assure complete combustion to prevent the formation of phosgene. An acid scrubber is necessary to remove the halo acids produced.
Properties of 1,2,4-Trichlorobenzene
| Melting point: | 16 °C(lit.) |
| Boiling point: | 214 °C(lit.) |
| Density | 1.454 g/mL at 25 °C(lit.) |
| vapor density | >6 (vs air) |
| vapor pressure | 1 mm Hg ( 40 °C) |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | 2-8°C |
| solubility | water: insoluble |
| form | Liquid |
| color | Clear |
| Odor | Characteristic aromatic odor |
| explosive limit | 6.6%, 150°F |
| Water Solubility | INSOLUBLE |
| λmax | λ: 308 nm Amax: 1.00 λ: 310 nm Amax: 0.50 λ: 350 nm Amax: 0.05 λ: 375-400 nm Amax: 0.01 |
| Merck | 14,9631 |
| BRN | 956819 |
| Henry's Law Constant | 0.997 at 20.0 °C (wetted-wall column, ten Hulscher et al., 1992) 1.24, 2.27, 2.58, 3.06, and 3.90 at 2.0, 6.0, 10.0, 18.0, and 25.0 °C, respectively (EPICS-SPME,
Dewulf et al., 1999) |
| Exposure limits | NIOSH REL: TWA ceiling 5 ppm (40 mg/m3); ACGIH TLV: ceiling 5 ppm
(adopted). |
| Dielectric constant | 2.2400000000000002 |
| Stability: | Stable. Incompatible with strong oxidizing agents. Combustible. |
| CAS DataBase Reference | 120-82-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzene, 1,2,4-trichloro-(120-82-1) |
| EPA Substance Registry System | 1,2,4-Trichlorobenzene (120-82-1) |
Safety information for 1,2,4-Trichlorobenzene
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for 1,2,4-Trichlorobenzene
1,2,4-Trichlorobenzene manufacturer
JSK Chemicals
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 1,2,4-Trichlorobenzene 98%View Details
1,2,4-Trichlorobenzene 98%View Details -
 1,2,4-Trichlorobenzene 98%View Details
1,2,4-Trichlorobenzene 98%View Details -
 1, 2, 4-Trichlorobenzene CAS 120-82-1View Details
1, 2, 4-Trichlorobenzene CAS 120-82-1View Details
120-82-1 -
 1, 2, 4-Trichlorobenzene CAS 120-82-1View Details
1, 2, 4-Trichlorobenzene CAS 120-82-1View Details
120-82-1 -
 1, 2, 4-Trichlorobenzene CAS 120-82-1View Details
1, 2, 4-Trichlorobenzene CAS 120-82-1View Details
120-82-1 -
 1 2 4 Trichlorobenzene, For Pharma / Industrial, Purity: 99%View Details
1 2 4 Trichlorobenzene, For Pharma / Industrial, Purity: 99%View Details
120-82-1 -
 1-2-4 Tri Chloro BenzeneView Details
1-2-4 Tri Chloro BenzeneView Details
120-82-1 -
 1 2 4 Trichlorobenzene, Grade: Industrial, Purity: 99View Details
1 2 4 Trichlorobenzene, Grade: Industrial, Purity: 99View Details
120-82-1
