3-Methylpiperidine
Synonym(s):β-Pipecoline;3-Pipecoline
- CAS NO.:626-56-2
- Empirical Formula: C6H13N
- Molecular Weight: 99.17
- MDL number: MFCD00005994
- EINECS: 210-953-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
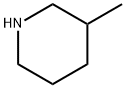
What is 3-Methylpiperidine?
Chemical properties
clear colorless to yellow liquid
The Uses of 3-Methylpiperidine
3-Methylpiperidine, is used as a reactant for synthesis of Phenylpropenamide derivatives for anti-hepatitis B virus activity, CB2 receptor agonists for treatment of chronic pain, aurora kinase inhibitors, spiroimidazolidinone NPC1L1 inhibitors, 1,3,5-Oxadiazinones, selective serotonin 5-HT6 receptor antagonists. It is also used as a pharmaceutical intermediate and as a intermediate in organic synthesis.
General Description
A colorless liquid with a characteristic odor. Less dense than water. Flash point less than 141°F. Contact may cause severe irritation to skin, eyes, and mucous membranes. Toxic by ingestion, inhalation and skin absorption.
Air & Water Reactions
Highly flammable. Water soluble.
Reactivity Profile
3-METHYLPIPERIDINE neutralizes acids to form salts plus water in exothermic reactions. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Generates flammable gaseous hydrogen in combination with strong reducing agents, such as hydrides.
Health Hazard
May cause toxic effects if inhaled or ingested/swallowed. Contact with substance may cause severe burns to skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
Flammable/combustible material. May be ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Purification Methods
Purify it via the hydrochloride (m 172o). The hydrobromide has m 162-163o(from iso-PrOH). [Chapman et al. J Chem Soc 1925 1959, Beilstein 20 III/IV 1499, 20/4 V 100.]
Properties of 3-Methylpiperidine
| Melting point: | -24 °C |
| Boiling point: | 125-126 °C/763 mmHg (lit.) |
| Density | 0.845 g/mL at 25 °C (lit.) |
| refractive index | n |
| Flash point: | 63 °F |
| storage temp. | Flammables area |
| form | clear liquid |
| pka | pK1:11.07(+1) (25°C) |
| color | Colorless to Light yellow to Light orange |
| Water Solubility | miscible |
| BRN | 79807 |
| CAS DataBase Reference | 626-56-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Piperidine, 3-methyl-(626-56-2) |
| EPA Substance Registry System | Piperidine, 3-methyl- (626-56-2) |
Safety information for 3-Methylpiperidine
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 3-Methylpiperidine
3-Methylpiperidine manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 626-56-2 3-Methyl-piperidine 98%View Details
626-56-2 3-Methyl-piperidine 98%View Details
626-56-2 -
 3-Methylpiperidine CAS 626-56-2View Details
3-Methylpiperidine CAS 626-56-2View Details
626-56-2 -
 3-Methylpiperidine CAS 626-56-2View Details
3-Methylpiperidine CAS 626-56-2View Details
626-56-2 -
 3-METHYLPIPERIDINE CAS 626-56-2View Details
3-METHYLPIPERIDINE CAS 626-56-2View Details
626-56-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1