3-Furoic acid
Synonym(s):Furan-3-carboxylic acid
- CAS NO.:488-93-7
- Empirical Formula: C5H4O3
- Molecular Weight: 112.08
- MDL number: MFCD00005350
- EINECS: 207-689-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
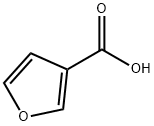
What is 3-Furoic acid?
Description
3-Furoic acid, also known as 3-carboxyfuran or 3-furoate, belongs to the class of organic compounds known as furoic acids. These are organic compounds containing a furoic acid moiety, with a structure characterized by a furan ring bearing a carboxylic acid group at the C2 or C3 carbon atom.
3-Furoic acid is an organic acid regularly occurring in urine of healthy individuals. It is used in synthesis of furan-2,5- and furan-2,4-dicarboxylic acid under solvent-free conditions via disproportionation reaction. It exhibits hypolipidemic activity in rodents. It lowers serum cholesterol and serum triglyceride levels in mice and rats.
Chemical properties
white to light yellow crystal powde
The Uses of 3-Furoic acid
3-Furoic acid can be used as a reactant to synthesize:
Furan-2,5- and furan-2,4-dicarboxylic acid under solvent-free conditions via disproportionation reaction.
(±)-Hyperolactone A by reacting with 2-methylbutanal.
Furo[2,3-b]pyridin-4-one-5-carboxylate ester derivatives as potential non-nucleoside inhibitors of human herpesvirus polymerases.
The Uses of 3-Furoic acid
3-Furoic acid is used in synthesis of furan-2,5- and furan-2,4-dicarboxylic acid under solvent-free conditions via disproportionation reaction. It exhibits hypolipidemic activity in rodents. It lowers serum cholesterol and serum triglyceride levels in mice and rats.
The Uses of 3-Furoic acid
3-Furoic acid can be used as a reactant to synthesize:
- Furan-2,5- and furan-2,4-dicarboxylic acid under solvent-free conditions via disproportionation reaction.
- (±)-Hyperolactone A by reacting with 2-methylbutanal.
- Furo[2,3-b]pyridin-4-one-5-carboxylate ester derivatives as potential non-nucleoside inhibitors of human herpesvirus polymerases.
Definition
ChEBI: A furoic acid carrying the carboxy group at position 3.
Synthesis Reference(s)
Tetrahedron Letters, 26, p. 1509, 1985 DOI: 10.1016/S0040-4039(00)98538-1
Biological Activity
3-Furoic acid exhibits hypolipidemic activity in rodents. It lowers serum cholesterol and serum triglyceride levels in mice and rats.
Biochem/physiol Actions
3-Furoic acid exhibits hypolipidemic activity in rodents. It lowers serum cholesterol and serum triglyceride levels in mice and rats.
Purification Methods
Crystallise the acid from water or aqueous EtOH, and sublime it in a vacuum. [Beilstein 18 I 439, 18 III/IV 4052, 18/6 V 196.]
Properties of 3-Furoic acid
| Melting point: | 120-122 °C (lit.) |
| Boiling point: | 229.64°C |
| Density | 1.3220 |
| refractive index | 1.4710 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 3.9(at 25℃) |
| color | Off-White to Beige |
| Water Solubility | insoluble |
| BRN | 108638 |
| CAS DataBase Reference | 488-93-7(CAS DataBase Reference) |
| NIST Chemistry Reference | 3-Furancarboxylic acid(488-93-7) |
Safety information for 3-Furoic acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 3-Furoic acid
| InChIKey | IHCCAYCGZOLTEU-UHFFFAOYSA-N |
3-Furoic acid manufacturer
HYPERSYNTH LIFE SCIENCES
HYCHEM LABORATORIES
New Products
1-Amino-1-cyclohexanecarboxylic acid 2-Hydroxyacetamide Ethyl 5-cyclopropyl-1H-pyrazole-3-carboxylate 2,4,6-Trichloroaniline ELECTROLYTIC IRON POWDER 1-Aminocyclobutanecarboxylic acid 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride Decanonitrile tert-butyl 4-(1H-benzo[d]iMidazol-2-yl)piperidine-1-carboxylate N,N'-diallyl-1,3-diaminopropanedihydrochloride 3-Iodo-6-nitroindazole (4-(4-Fluorophenoxy)phenyl)methanamine Tert-butyl N-(pent-4-yn-2-yl) carbamate 2-Chloro-3-nitropyridine 5-Bromo-2,3-dimethoxypyridine 2-chloro-4-methyl-5-nitro pyridine 2,5-dibromopyridine terephthalonitrile 5-Bromo-4-chloro-2(1h)-pyridinone, 5-Fluoro-2-Oxindole N, N-Carbonyldiimidazole (CDI) 5-Cyanophthalide 10-Methoxy-5H-dibenz[b,f]azepine 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA]Related products of tetrahydrofuran


You may like
-
 3-Furoic acid 488-93-7 98%View Details
3-Furoic acid 488-93-7 98%View Details
488-93-7 -
 3-Furoic acid 98%View Details
3-Furoic acid 98%View Details -
 3-Furoic acid CAS 488-93-7View Details
3-Furoic acid CAS 488-93-7View Details
488-93-7 -
 3-Furoic Acid pure CAS 488-93-7View Details
3-Furoic Acid pure CAS 488-93-7View Details
488-93-7 -
 Furan-3-carboxylic acid 98% CAS 488-93-7View Details
Furan-3-carboxylic acid 98% CAS 488-93-7View Details
488-93-7 -
 3-Furoic acid 98%View Details
3-Furoic acid 98%View Details -
 3-Furoic acid CAS 488-93-7View Details
3-Furoic acid CAS 488-93-7View Details
488-93-7 -
 3-Furancarboxylic Acid CAS 488-93-7View Details
3-Furancarboxylic Acid CAS 488-93-7View Details
488-93-7