Calcium formate
Synonym(s):Formic acid calcium salt
- CAS NO.:544-17-2
- Empirical Formula: C2H2CaO4
- Molecular Weight: 130.11
- MDL number: MFCD00036108
- EINECS: 208-863-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-31 18:46:13
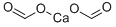
What is Calcium formate ?
Description
Calcium formate is a white to almost white fine crystalline powder. It can be used an accelerator for the pozzolanic cement pastes. On the one hand, it shortens the initial and final setting times and increases the compressive strength and combined water content as well as gel/space ratio at all ages of hydration. On the other hand, it decreases the total porosity. It has been shown that it has a growth-promoting effect in weanling pigs challenged with E. coli, independently of their susceptibility to the intestinal adhesion of this strain. More importantly, calcium formate can be used as a nutrient supplement to the feed of young growing pigs or fattening poultry, further boosting the growth of animals and the feed utilization. At the same time, it causes a reduction of the occurrence of piglet diarrhea.

Chemical properties
Calcium formate, Ca(HCOO)2, is the calcium salt of formic acid, HCOOH. It is also known as food additive E238 in food industry. The mineral form is very rare and called formicaite. It is known from a few boron deposits. It may be produced synthetically by reacting calcium oxide or calcium hydroxide with formic acid.
Chemical properties
Calcium formate is white solid, solubility at 0 °C 13.90 g, at 40 °C 14.56 g, at 80 °C 15.22 g of anhydrous salt per 100 g saturated solution, formed by reaction of calcium carbonate or hydroxide and formic acid. Calcium formate, when heated with a calcium salt of a carboxylic acid higher in the series, yields an aldehyde.
The Uses of Calcium formate
Preservative for food, silage; as binder for fine-ore briquets; in drilling fluids and lubricants.
Calcium formate is an organic salt suitable for use in pig and poultry diets. It acts as a feedstock preservative and has an acidifying effect on the gastro-intestinal tract, which promotes good gut-health. The efficiency of pig and poultry feedstock digestion is dependent on the indigenous micro-organism concentration in the gastro-intestinal tract. With the approaching ban on the use of prophylactic antibiotics in animal feed as a means to control disease and promote growth, alternatives are needed to limit the proliferation of pathogenic bacteria in the gut which can impair feedstock digestion as well as cause enteric diseases such as E. Coli and Salmonella. Acidifiers such as calcium formate preserve the feedstock before consumption and lower the pH in the gastro-intestinal tract, creating unfavourable conditions for these bacteria to grow.
Calcium formate is used extensively in the leather industry as a masking agent in the chrome-tanning process. The addition of calcium formate to the tannage formulation promotes faster, more efficient penetration of the chrome in the leather. Calcium formate can also be used as a replacement for formic acid in the pickling operation.
The Uses of Calcium formate
Calcium Formate is Hydrophobic additives for construction materials.Formic Acid is used as a potential energy source in the preparation of fuel cells. Also used in chemical synthesis of various anti-inflammatory and anti-microbial agents.
Definition
ChEBI: Calcium formate is an organic molecular entity.
Reactions
Calcium formate can be used to prepare solutions of other water-soluble formate salts. For example, the addition of nickel (II) sulfate to a solution of calcium formate results in the precipitation of insoluble calcium sulfate, leaving nickel (II) formate in solution:
Ca(OOCH)2 + NiSO1 → CaSO4 + Ni(OOCH)2
In a similar reaction, sulfuric acid reacts with calcium formate to provide low-cost solutions of formic acid:
Ca(OOCH)2 + H2 SO4 → CaSO4 + 2HCOOH
When heated, calcium formate decomposes to calcium carbonate and formaldehyde:
Ca(OOCH)2 → CaCO3 + HCHO
Calcium formate can be used as a low-cost reducing agent in the conversion of carboxylic acids to aldehydes. This process is an effective substitute for the troublesome Rosenmund reduction.
(RCOO)2Ca + (HCOO)2CA → 2RCH + 2CaCO3
Flammability and Explosibility
Not classified
Safety Profile
Poison by intravenous route. Moderately toxic by ingestion. An eye irritant. When heated to decomposition it emits acrid smoke and fumes. See also CALCIUM COMPOUNDS.
Purification Methods
Recrystallise it from water (5mL/g) by partial evaporation in a desiccator. [Beilstein 2 IV 16.]
Toxicity evaluation
Calcium formate has a low order of toxicity. In rats, the acute oral LD50 is 2650mg/kg; by intravenous injection, the LD50 is 154mg/kg.
In a study with rats, calcium formate added to the drinking water at 0.2% for 3 years or 0.4% for 2 years didn't affect growth, fertility or function in up to 5 generations.
Calcium formate would be expected to be irritating to the eyes and skin. In case of eye contact, immediately flush the eyes with water for 15 minutes. Avoid prolonged or repeated exposure of the skin.
References
Heikal, Mohamed. "Effect of calcium formate as an accelerator on the physicochemical and mechanical properties of pozzolanic cement pastes." Cement & Concrete Research 34.6(2004):1051-1056.
Bosi, P, et al. "The influence of fat protection of calcium formate on growth and intestinal defense in Escherichia coli K88-challenged weanling pigs." Animal Feed Science & Technology 139.3–4(2007):170-185.
Bolze, Rudolf, et al. "Agent for increasing the performance of pigs and poultry." EP, EP0260391. 1988.
Properties of Calcium formate
| Melting point: | 300 °C |
| Density | 2,02 g/cm3 |
| vapor pressure | 3.41Pa at 25℃ |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | orthorhombic crystals |
| pka | 3.8[at 20 ℃] |
| Specific Gravity | 2.02 |
| color | orthorhombic crystals, crystalline |
| Odor | wh. orthorhombic cryst. or cryst. powd., sl. acetic acid-like odor |
| PH | 7.51(1 mM solution);7.92(10 mM solution);8.24(100 mM solution);8.47(1000 mM solution) |
| Water Solubility | SOLUBLE |
| Hydrolytic Sensitivity | 0: forms stable aqueous solutions |
| λmax | λ: 260 nm Amax: ≤0.04 λ: 280 nm Amax: ≤0.03 |
| Merck | 13,1670 |
| BRN | 3624099 |
| CAS DataBase Reference | 544-17-2(CAS DataBase Reference) |
| EPA Substance Registry System | Formic acid, calcium salt (544-17-2) |
Safety information for Calcium formate
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for Calcium formate
| InChIKey | CBOCVOKPQGJKKJ-UHFFFAOYSA-L |
Calcium formate manufacturer
Zama Chemical
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 CALCIUM FORMATE 99%View Details
CALCIUM FORMATE 99%View Details -
 Calcium formate 99%View Details
Calcium formate 99%View Details -
 Calcium formate CAS 544-17-2View Details
Calcium formate CAS 544-17-2View Details
544-17-2 -
 Powder White Crystal Calcium Formate CAS Number: 544-17-2View Details
Powder White Crystal Calcium Formate CAS Number: 544-17-2View Details
544-17-2 -
 Calcium Formate PowderView Details
Calcium Formate PowderView Details
544-17-2 -
 Calcium Formate ., 99%, Technical GradeView Details
Calcium Formate ., 99%, Technical GradeView Details
544-17-2 -
 Calcium Formate, Packaging: 25 kgView Details
Calcium Formate, Packaging: 25 kgView Details
544-17-2 -
 Powder Calcium Formate, 25View Details
Powder Calcium Formate, 25View Details
544-17-2
