2-Furoic acid
Synonym(s):Furan-2-carboxylic acid
- CAS NO.:88-14-2
- Empirical Formula: C5H4O3
- Molecular Weight: 112.08
- MDL number: MFCD00003238
- EINECS: 201-803-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
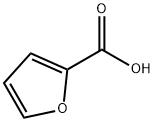
What is 2-Furoic acid?
Description
2-Furoic acid is an organic compound, consisting of a furan ring and a carboxylic acid side-group. Along with other furans, its name is derived from the Latin word furfur, meaning bran, from which these compounds were first produced. The salts and esters of furoic acids are known as furoates. 2-Furoic acid is most widely encountered in food products as a preservative and a flavouring agent, where it imparts a sweet, earthy flavour.
Chemical properties
white to light yellow crystal powder. 1g is dissolved in 26ml cold water or 4ml boiling water, easily soluble in ethanol and ether. Sublimation at 130-140°C (6.65-8kPa).
The Uses of 2-Furoic acid
2-Furoic acid is an heterocyclic carboxylic acid that is widely used in food products as a preservative and flavouring agent. It is also used in the synthesis of methylfuran, furoamide and furoic acid esters and salts. In the plastics industry, it can be used as plasticizer, thermosetting resin, etc. substitute for chloropicrin in disinfecting grain elevators.
Definition
ChEBI: 2-furoic acid is a furoic acid having the carboxylic acid group located at position 2. It has a role as an inhibitor, a human xenobiotic metabolite, a Saccharomyces cerevisiae metabolite, a plant metabolite and a bacterial xenobiotic metabolite. It is a conjugate acid of a 2-furoate.
Preparation
2-furoic acid can be synthesized by the oxidation of furfural. The reaction was carried out at 45°C with vigorous stirring. The reactant is filtered to recover the catalyst copper oxide, the filtrate is acidified with 50% sulfuric acid to pH 3-2.5, suction filtered, washed with water, and dried to obtain 2-furoic acid.
What are the applications of Application
2-Furoic acid (FA) is a preservative, acting as a bactericide and fungicide. Some of the applications of 2-furoic acid include:
Synthesis of a single-molecule magnet, Mn11Gd2 based high-nuclearity heterometallic complex.
Synthesis of orally active antidiabetic vanadyl complex, bis(α-furancarboxylato)oxovanadium(IV).
As a ligand in the synthesis of luminescent 4f-3d heterometallic one-dimensional coordination polymers.
Synthesis of biocompatible multifunctional dextran furoate nanospheres.
FA is often used as a starting material for the production of furoate esters.
FA and its derivatives are also used in the production of nylons principally applied in biomedical researches.
Biological Functions
2-Furoic acid was shown to effectively lower serum cholesterol and serum triglyceride levels significantly in rats with an elevation of HDL cholesterol level at 20 mg/kg/day orally. 2-furoic acid appears to interfere directly with the activity of intracellular enzymes rather than affecting high affinity-mediated lipoprotein membrane receptors. In vivo, treatment with 2-furoic acid led to a reduction in the liver and small intestine ATP-dependent citrate lyase, acetyl CoA synthetase, acyl CoA cholesterol acyl transferase, sn-glycerol 3-phosphate acyl transferase, phosphatidylate phosphohydrolase, and heparin induced lipoprotein lipase activities. 2-Furoic acid reduced biliary cholesterol levels, but the agent increased bile salts, which are lithogenic. Acute toxicity studies in mice suggest that the agent has some hepatic toxicity effects. The LD50 was relatively low at 250 mg/kg IP in mice[1].
Hazard
Strong irritant to eyes and skin.
Flammability and Explosibility
Not classified
Solubility in organics
Soluble in alcohol, miscible with oils.
Purification Methods
Crystallise the acid from hot water (charcoal), dry it at 120o for 2hours, then recrystallise it from CHCl3, and again dry it at 120o for 2hours. For use as a standard in volumetric analysis, good quality commercial acid should be crystallised from CHCl3 and dried as above or sublimed at 130-140o/50-60mm or less. [Beilstein 18 I 438, 18 II 265, 18 III/IV 3914, 18/6 V 102.]
References
[1] Iris H. Hall. “Hypolipidemic Effects of 2-Furoic Acid in Sprague-Dawley Rats.” Archiv der Pharmazie 326 1 (1993): 15–23.
Properties of 2-Furoic acid
| Melting point: | −2 °C(lit.) |
| Boiling point: | 173-174 °C(lit.) |
| Density | 1.324 g/mL at 25 °C(lit.) |
| vapor pressure | 13.732Pa at 25℃ |
| refractive index | n |
| Flash point: | 185 °F |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | 37g/l |
| form | Fine Crystalline Powder |
| pka | 3.16(at 25℃) |
| color | Off-white to beige |
| Odor | odorless |
| Water Solubility | 36 g/L (20 ºC) |
| Merck | 14,4307 |
| BRN | 110149 |
| CAS DataBase Reference | 88-14-2(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Furancarboxylic acid(88-14-2) |
| EPA Substance Registry System | 2-Furancarboxylic acid (88-14-2) |
Safety information for 2-Furoic acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-Furoic acid
| InChIKey | SMNDYUVBFMFKNZ-UHFFFAOYSA-N |
2-Furoic acid manufacturer
Galaxy Laboratories Private Limited
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2-FUROIC ACID 99%View Details
2-FUROIC ACID 99%View Details -
 2-Furoic acid 98%View Details
2-Furoic acid 98%View Details -
 2-Furoic Acid pure CAS 88-14-2View Details
2-Furoic Acid pure CAS 88-14-2View Details
88-14-2 -
 2-Furoic acid CAS 88-14-2View Details
2-Furoic acid CAS 88-14-2View Details
88-14-2 -
 2-Furancarboxylic Acid CAS 88-14-2View Details
2-Furancarboxylic Acid CAS 88-14-2View Details
88-14-2 -
 2-Furoic acid 88-14-2 98%View Details
2-Furoic acid 88-14-2 98%View Details
88-14-2 -
 2-Furoic acid CAS 88-14-2View Details
2-Furoic acid CAS 88-14-2View Details
88-14-2 -
 2-Furoic acid CAS 88-14-2View Details
2-Furoic acid CAS 88-14-2View Details
88-14-2