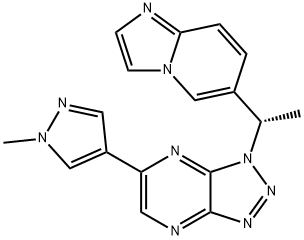
3-(5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1-yl)-4-(1H-indol-3-yl)-pyrrolidine-2,5-dione
- CAS NO.:905854-02-6
- Empirical Formula: C23H19N3O2
- Molecular Weight: 369.42
- MDL number: MFCD11977597
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
![3-(5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1-yl)-4-(1H-indol-3-yl)-pyrrolidine-2,5-dione Structural](https://img.chemicalbook.in/CAS/GIF/905854-02-6.gif)
What is 3-(5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1-yl)-4-(1H-indol-3-yl)-pyrrolidine-2,5-dione?
The Uses of 3-(5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1-yl)-4-(1H-indol-3-yl)-pyrrolidine-2,5-dione
Tivantinib is a hepatocyte growth factor receptor (proto-oncogene c-Met; MET) inhibitor. Tivantinib inhibits human c-Met receptor tyrosine kinase selectively and is a promising therapeutic option fo r the treatment of c-Met-associated cancers.
The Uses of 3-(5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1-yl)-4-(1H-indol-3-yl)-pyrrolidine-2,5-dione
Tivantinib is a hepatocyte growth factor receptor (proto-oncogene c-Met; MET) inhibitor. Tivantinib inhibits human c-Met receptor tyrosine kinase selectively and is a promising therapeutic option for the treatment of c-Met-associated cancers. Potent c-MET inhibitor.
What are the applications of Application
ARQ 197 is a novel and selective human Met inhibitor (IC50 = 0.1 μM)
Definition
ChEBI: LSM-1131 is a member of indoles.
Biological Activity
tivantinib (arq 197) is an oral, non–adenosine triphosphate-competitive, selective, small-molecule met proto-oncogene (c-met) inhibitor. the calculated inhibitory constant (ki) for tivantinib to inhibit recombinant human c-met was approximately 355 nmol/l.c-met, a type of receptor tyrosine kinase, is a high-affinity receptor of the hepatocyte growth factor (hgf). dysregulated hgf/c-met-signaling pathway frequently occurs in human cancer [1].tivantinib had weak inhibitory effects on vegf receptor-3 (flt4), p21-activated kinase 3, calmodulin-dependent kinase ii delta, and pim-1 [1]. tivantinib displayed cytotoxic activity against a wide panel of human tumor cell lines with an ec50 ranging from 300-600 nmol/l [4]. remarkably, a549, h3122, pc9 (del e746_a750), pc9 gr4 (del e746_a750/t790m), hcc827, hcc827 gr6, h1993 and ebc-1 cell lines showed some degree of sensitivity to tivantinib, with ic50s ranging between 0.36 and 0.8 μm [5]. in tumor cell lines, gtl-16, mkn-45, hs746t, snu-5, ebc-1, h1993, nci-h441, a549, hct-116, u87-mg, a2780, and tov-112d, tivantinib indiscriminately inhibited cell proliferation independently of c-met gene amplification and met protein expression with an ec50 ranging from 60 to 600 nmol/l. further research showed that tivantinib promotes mitotic arrest, prevents cells from re-entering g1, and drives them to apoptosis, and induces programmed cell death regardless of the presence or absence of a functional met kinase [4].tivantinib has demonstrated antitumor activity in a wide range of human tumor cell lines and in xenograft models of human lung, colon, prostate, pancreas, and breast cancer [1] [2] [3]. female 4-week-old athymic nude (nu/nu) mice were used as experimental animals. tivantinib at a dose of 120 mg/kg significantly inhibited tumor burden in the bone of treated animals compared with the controls, starting from 14 to 21 days after cell injection. increasing doses of tivantinib decreased the number and the extent of osteolytic lesions [6].
References
[1]. ryohei katayama, aki aoyama, takao yamori, et al. cytotoxic activity of tivantinib (arq 197) is not due solely to c-met inhibition. cancer research, 2013, 73(10): 3087-3097.
[2]. andrew j.wagner, john m. goldberg, steven g. dubois, et al. tivantinib (arq 197), a selective inhibitor of met, in patients with microphthalmia transcription factor–associated tumors. cancer, 2012: 5894-5902.
[3]. n. yamamoto, h. murakami, t. nishina, et al. the effect of cyp2c19 polymorphism on the safety, tolerability, and pharmacokinetics of tivantinib (arq 197): results from a phase i trial in advanced solid tumors. annals of oncology, 2013, 00: 1–7.
[4]. cristina basilico, selma pennacchietti, elisa vigna, et al. tivantinib (arq197) displays cytotoxic activity that is independent of its ability to bind met. clin cancer res, 2013, 19(9):2381-92.
[5]. cristina basilico, selma pennacchietti, elisa vigna, et al. tivantinib (arq197) displays cytotoxic activity that is independent of its ability to bind met. clinical cancer research, 2013, 19(9): 2381–92.
[6]. sara previdi, giovanni abbadessa, francesca dalò, et al. breast cancer–derived bone metastasis can be effectively reduced through specific c-met inhibitor tivantinib (arq 197) and shrna c-met knockdown. mol cancer ther, 2011, 11(1):214-23.
Properties of 3-(5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1-yl)-4-(1H-indol-3-yl)-pyrrolidine-2,5-dione
| Boiling point: | 715.9±60.0 °C(Predicted) |
| Density | 1.49 |
| storage temp. | -20°C Freezer, Under inert atmosphere |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 9.28±0.70(Predicted) |
| color | Light Orange |
Safety information for 3-(5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1-yl)-4-(1H-indol-3-yl)-pyrrolidine-2,5-dione
Computed Descriptors for 3-(5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1-yl)-4-(1H-indol-3-yl)-pyrrolidine-2,5-dione
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Tivantinib >95% CAS 905854-02-6View Details
Tivantinib >95% CAS 905854-02-6View Details
905854-02-6 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1