3-(4-METHYLBENZYLIDENE)CAMPHOR
Synonym(s):1,7,7-Trimethyl-3-(4-methylbenzylidene)-norbornan-2-one;1,7,7-Trimethyl-3-[(4-methylphenyl)methylene]-bicyclo[2.2.1]heptan-2-one;3-(4-Methylbenzylidene)camphor;4-MBC;4-Methylbenzylidene camphor
- CAS NO.:36861-47-9
- Empirical Formula: C18H22O
- Molecular Weight: 254.37
- MDL number: MFCD00209662
- EINECS: 253-242-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:40
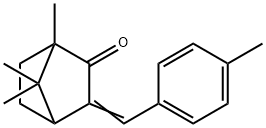
What is 3-(4-METHYLBENZYLIDENE)CAMPHOR?
Absorption
The maximum plasma concentration of enzacamene was 16ng/mL in healthy female volunteers following daily whole-body topical application of 2mg/cm^2 of sunscreen formulation at 10% (weight/weight) for four days . Blood concentration of enzacamene (4-MBC) and its main metabolite, 3-(4-carboxybenzylidene)camphor, peaked within 10 h after oral administration of enzacamene .
Toxicity
Oral LD50 and dermal LD50 in rat are reported to be 10,000 mg/kg . Oral TDLO in rat is 7 mg/kg . Oral and subcutaneous TDLO following continuous administration in rat are 476 mg/kg/4D and 4 mg/kg/2D, respectively . Cases of overdose have not been reported for enzacamene. Enzacamene is reported to be an endocrine disruptor that alters the reproductive axis.
Chemical properties
White Solid
The Uses of 3-(4-METHYLBENZYLIDENE)CAMPHOR
3-(4-Methylbenzylidene)camphor is an organic camphor derivative that is used in the cosmetic industry for its ability to protect the skin against UV, specifically UV B radiation. 4-Methylbenzylidene camphor(4-MBC) a UV-B ray filter, is an endocrine disruptors (ED).
The Uses of 3-(4-METHYLBENZYLIDENE)CAMPHOR
4-(Methylbenzylidene)camphor (4-MBC), a UV-B ray filter, is an endocrine disruptors (ED).
The Uses of 3-(4-METHYLBENZYLIDENE)CAMPHOR
3-(4-Methylbenzyliden)camphor is an UV-B absorbing agent in sunscreen cosmetics of the type creams, lotions, lipsticks, sun oils, etc.
Indications
Indicated for use as an active sunscreen agent.
Background
Commonly known as 4-methylbenzylidene-camphor (4-MBC), enzacamene is a camphor derivative and an organic chemical UV-B filter. It is used in cosmetic products such as sunscreen to provide skin protection against UV rays. While its effects on the human reproductive system as an endocrine disruptor are being investigated, its use in over-the-counter and cosmetic products is approved by Health Canada. Its tradenames include Eusolex 6300 (Merck) and Parsol 5000 (DSM).
What are the applications of Application
3-(4-Methylbenzylidene)camphor is a UV-B ray filter
Biological Activity
4-methylbenzylidene camphor (4-mbc) is an ultraviolet light blocker used in cosmetics and sunscreen preparations that also has estrogenic activities.since the estrogen receptor (er) ligand type can influence transactivation, it is important to obtain information on molecular actions of nonclassical er agonists.
Pharmacokinetics
Several studies suggest that enzacamene elicit estrogen-like effects. In prepubertal male rats exposed to enzacamene during embryonic and fetal development, decrease in testicular weight with decreased levels of LH, GnRH, and glutamate were observed; in comparison, there was an increase in LH, GnRH, and aspartate levels in peripubertal rats . These findings suggest that high concentrations of enzacamene during embryonic and fetal stage inhibits the testicular axis in male rats during the prepubertal stage and stimulates it during peripubertad stage . In a study of zebrafish (Danio rerio) embryo, exposure to enzacamene during early vertebrate development was associated with muscular and neuronal defects that may result in developmental defects, including a reduction in AChE activity, disorganized pattern of slow muscle fibers, and axon pathfinding errors during motor neuron innervation . Enzacamene displays a weak binding activity in receptors binding assays using the mammalian estrogen receptor (ER) .
in vitro
previous results of competitive binding assays using cytosolic protein preparations from xenopus hepatocytes demonstrated that 4-mbc could weakly bind to the er. in addition, 4-mbc at a 100 micromol/l was not able to replace estradiol from the receptor completely [1].
in vivo
the estrogen target gene expression in uterus of long evans rats after exposure to 4-mbc was studied. results showed that 4-mbc could altere steady-state levels of mrnas encoding for er, progesterone receptor (pr), and androgen recepto in uterus of 12-wk-old offspring. to evaluate sensitivity to estradiol (e2), offspring were injected with e2, and killed 6 h later. acute up-regulation of pr and igf-i and down-regulation of er and androgen receptor by e2 were reduced in 4-mbc treated rats dose-dependently [2].
Metabolism
Based on the findings of a rat pharmacokinetic study, it is proposed that absorbed enzacamene following oral administration undergo extensive first-pass hepatic metabolism . Following oral administration of enzacamene (4-MBC) in rats, detected metabolites in the plasma and urine were 3-(4-carboxybenzylidene)camphor and as four isomers of 3-(4-carboxybenzylidene)hydroxycamphor containing the hydroxyl group located in the camphor ring system with 3-(4-carboxybenzylidene)-6-hydroxycamphor as the major metabolite. However the blood concentrations of 3-(4-carboxybenzylidene)-6-hydroxycamphor were below the limit of detection following peak concentration .
Via hydroxylation mediated by cytochrome P450 system, 3-(4-hydroxymethylbenzylidene)camphor is formed. This metabolite is further oxidized to 3-(4-carboxybenzylidene)camphor via oxidation of alcohol dehydrogenase and aldehyde dehydrogenase, and may be further hydroxylated to form 3-(4-carboxybenzylidene)-6-hydroxycamphor mediated by CYP450 system .
References
[1] klann a, levy g, lutz i, müller c, kloas w, hildebrandt jp. estrogen-like effects of ultraviolet screen 3-(4-methylbenzylidene)-camphor (eusolex 6300) on cell proliferation and gene induction in mammalian and amphibian cells. environ res. 2005 mar;97(3):274-81.
[2] s. durrer, k. maerkel, m. schlumpf, et al. estrogen target gene regulation and coactivator expression in rat uterus after developmental exposure to the ultraviolet filter 4-methylbenzylidene camphor. endocrinology 146(5), 2130-2139 (2005).
[3] n. r. janjua, b. mogensen, a. m. andersson, et al. systemic absorption of the sunscreens benzophenone-3, octyl-methoxycinnamate, and 3-(4-methyl-benzylidene) camphor after whole-body topical application and reproductive hormone levels in humans. journal of investigative dermatology 123, 57-61(2004).
Properties of 3-(4-METHYLBENZYLIDENE)CAMPHOR
| Melting point: | 66-68°C |
| Boiling point: | 198-200 °C |
| Density | 1.064±0.06 g/cm3(Predicted) |
| RTECS | DT5099565 |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | insoluble in H2O; ≥10 mg/mL in DMSO; ≥111.2 mg/mL in EtOH |
| form | neat |
| form | Solid |
| color | White to off-white |
| BRN | 9213949 |
| CAS DataBase Reference | 36861-47-9(CAS DataBase Reference) |
| EPA Substance Registry System | Bicyclo[2.2.1]heptan-2-one, 1,7,7-trimethyl-3-[(4-methylphenyl)methylene]- (36861-47-9) |
Safety information for 3-(4-METHYLBENZYLIDENE)CAMPHOR
| Signal word | Warning |
| Pictogram(s) |
 Environment GHS09 |
| GHS Hazard Statements |
H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P501:Dispose of contents/container to..… |
Computed Descriptors for 3-(4-METHYLBENZYLIDENE)CAMPHOR
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
![(-)-2-[METHYLAMINO]-1-PHENYLPROPANE](https://img.chemicalbook.in/CAS/GIF/33817-09-3.gif)
You may like
-
 Methyl benzylidene camphor CAS 36861-47-9View Details
Methyl benzylidene camphor CAS 36861-47-9View Details
36861-47-9 -
 3-(4-Methylbenzylidene)camphor CAS 36861-47-9View Details
3-(4-Methylbenzylidene)camphor CAS 36861-47-9View Details
36861-47-9 -
 3-(4-Methylbenzylidene)camphor CAS 36861-47-9View Details
3-(4-Methylbenzylidene)camphor CAS 36861-47-9View Details
36861-47-9 -
 Methyl Benzylidene Camphor CAS 36861-47-9View Details
Methyl Benzylidene Camphor CAS 36861-47-9View Details
36861-47-9 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
