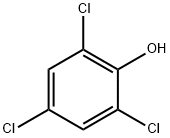
2,4,5-Trichlorophenol
- CAS NO.:95-95-4
- Empirical Formula: C6H3Cl3O
- Molecular Weight: 197.45
- MDL number: MFCD00002170
- EINECS: 202-467-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
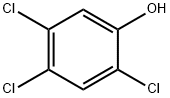
What is 2,4,5-Trichlorophenol?
Chemical properties
White to pale brown solid in appearance; 2,4,5-Trichlorophenol also looks like small needles. It has a really strong odor that smells like phenol (a poisonous crystal-looking compound). This man-made substance is not found naturally in the environment.
Physical properties
Colorless crystals or yellow to gray flakes with a strong, disinfectant or phenolic odor. At 40 °C, the average odor threshold concentration and the lowest concentration at which an odor were detected were 350 and 63 μg/L, respectively. At 25 °C, the lowest concentration at which a taste was detected was 100 μg/L (Young et al., 1996).
The Uses of 2,4,5-Trichlorophenol
2,4,5-Trichlorophenol is used as a broad range pesticide against insects, fungi, vegetation and bacteria. It has become a common environmental contaminant and probable human carcinogen.
Definition
ChEBI: 2,4,5-trichlorophenol is a trichlorophenol carrying chloro groups at positions 2, 4 and 5.
Preparation
2,4,5-Trichlorophenol is prepared indirectly, by the alkaline hydrolysis of 1,2,4,5-tetrachlorobenzene,because the direct chlorination of 2,5-dichlorophenol, difficult to achieve, proceeds with poor yield.
General Description
Colorless needles, gray flakes or off-white lumpy solid. Phenolic odor. Formerly used as a fungicide and bactericide.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
2,4,5-Trichlorophenol is a weak monobasic acid. Incompatible with acid chlorides, acid anhydrides and oxidizing agents. Produces dioxin in alkaline medium at high temperatures
Health Hazard
If your skin comes into contact with 2,4,5-trichlorophenol, it may burn the skin and produce redness and edema in humans. It also irritates the eyes, nose, pharynx, and lungs in humans.
Tests involving acute exposure of rats, mice, and guinea pigs have demonstrated 2,4,5-trichlorophenol to havemoderateacute toxicity by oral exposure.
Fire Hazard
Literature sources indicate that 2,4,5-Trichlorophenol is nonflammable.
Safety Profile
Suspected carcinogen with experimental neoplastigenic data. Poison by intraperitoneal and intravenous routes. Moderately toxic by ingestion and subcutaneous routes. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of Cland explodes. See also CHLOROPHENOLS.
Environmental Fate
Biological. Chloroperoxidase, a fungal enzyme isolated from Caldariomyces fumago,
chlorinated 2,4,5-trichlorophenol to give 2,3,4,6-tetrachlorophenol (Wannstedt et al., 1990).
Photolytic. When 2,4,5-trichlorophenol (100 μM) in an oxygenated, titanium dioxide (2 g/L)
suspension was irradiated by sunlight (λ ≥340 nm), complete mineralization to carbon dioxide and
water and chloride ions was observed (Barbeni et al., 1987a).
The following phototransformation half-lives were reported for 2,4,5-trichlorophenol in
estuarine water exposed to sunlight and microbes: 1 h during winter; in distilled water: 0.6 and 1 h
during summer and winter, respectively; in poisoned estuarine water: 14 and 24 h during summer
and winter, respectively (Hwang et al., 1986).
A photooxidation rate constant of <3,000/M·sec was reported for the reaction of 2,4,5-
trichlorophenol and ozone in water at a pH range of 1.2 to 1.5 (Hoigné and Bader, 1983).
Chemical/Physical. 2,4,5-Trichlorophenol will not hydrolyze because it does not contain a
hydrolyzable functional group (Kollig, 1993).
During the manufacture/synthesis of 2,4,5-T using alkalies at high temperatures, some TCDD
may form (Worthing and Hance, 1991).
Purification Methods
Crystallise the phenol from EtOH or pet ether. [Beilstein 6 IV 962.]
Properties of 2,4,5-Trichlorophenol
| Melting point: | 64-67 °C
67-69 °C (lit.) |
| Boiling point: | 248 °C/740 mmHg (lit.) |
| Density | 1,678 g/cm3 |
| vapor pressure | 3.5 at 8 °C, 22 at 25 °C (quoted, Leuenberger et al., 1985a) |
| refractive index | 1.5300 (estimate) |
| Flash point: | 253°C |
| storage temp. | 2-8°C |
| solubility | Soluble in ethanol and ligroin (U.S. EPA, 1985) |
| form | Powder |
| pka | pK1:7.37 (25°C) |
| color | Colorless needles or gray flakes from pet ether |
| Odor | strong phenolic odor |
| Water Solubility | 947.8mg/L(25 ºC) |
| Merck | 14,9643 |
| BRN | 607569 |
| Henry's Law Constant | 1.76 at 25 °C (estimated, Leuenberger et al., 1985a) |
| Stability: | Stable. Note that this material creates dioxin in alkaline media at elevated temperatures. |
| CAS DataBase Reference | 95-95-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Phenol, 2,4,5-trichloro-(95-95-4) |
| EPA Substance Registry System | 2,4,5-Trichlorophenol (95-95-4) |
Safety information for 2,4,5-Trichlorophenol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2,4,5-Trichlorophenol
2,4,5-Trichlorophenol manufacturer
Chirag Organics Pvt Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 95-95-4 2,4,5-trichloro phenol 99%View Details
95-95-4 2,4,5-trichloro phenol 99%View Details
95-95-4 -
 2,4,5-Trichlorophenol CAS 95-95-4View Details
2,4,5-Trichlorophenol CAS 95-95-4View Details
95-95-4 -
 2,4,5-Trichlorophenol CAS 95-95-4View Details
2,4,5-Trichlorophenol CAS 95-95-4View Details
95-95-4 -
 2,4,5-Trichlorophenol CAS 95-95-4View Details
2,4,5-Trichlorophenol CAS 95-95-4View Details
95-95-4 -
 2,4,5-Trichlorophenol CAS 95-95-4View Details
2,4,5-Trichlorophenol CAS 95-95-4View Details
95-95-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8