2,4-Dichlorophenol
Synonym(s):2,4-DCP;2,4-Dichlorophenol
- CAS NO.:120-83-2
- Empirical Formula: C6H4Cl2O
- Molecular Weight: 163
- MDL number: MFCD00002169
- EINECS: 204-429-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
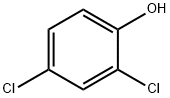
What is 2,4-Dichlorophenol?
Description
2,4-DCP is a colorless crystalline solid with acharacteristic odor. Molecular weight=163.00; Boilingpoint=210℃; Freezing/Melting point=45.0℃; Flashpoint=113℃. Hazard Identification (based on NFPA-704M Rating System): Health 1, Flammability 1, Reactivity 0.Slightly soluble in water.
Chemical properties
white to beige crystalline solid
Chemical properties
2,4-DCP is a colorless crystalline solid with a characteristic odor
Physical properties
Colorless to yellow crystals with a sweet, musty, or medicinal odor. At 40 °C, the average odor threshold concentration and the lowest concentration at which an odor was detected were 29 and 5.4 μg/L, respectively. Similarly, at 25 °C, the average taste threshold concentration and the lowest concentration at which a taste was detected were 2.5 and 0.98 μg/L, respectively (Young et al., 1996).
The Uses of 2,4-Dichlorophenol
2,4-Dichlorophenol is a chlorinated derivative of phenol and is used as an intermediate for the preparation of herbicide 2,4-dichlorophenoxyacetic acid (D435680).
The Uses of 2,4-Dichlorophenol
Intermediate in production of herbicidal chlorophenoxy acids such as 2,4- dichlorophenoxyacetic acid
Definition
ChEBI: A dichlorophenol that is phenol carrying chloro substituents at positions 2 and 4.
Synthesis Reference(s)
Synthetic Communications, 20, p. 2991, 1990 DOI: 10.1080/00397919008051517
General Description
Colorless crystalline solid with a medicinal odor. Melting point 45°C. Sinks in water. Strong irritant to tissues; toxic by ingestion.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
2,4-Dichlorophenol can react vigorously with oxidizing agents. Can also react with acids or acid fumes. Incompatible with acid chlorides and acid anhydrides.
Health Hazard
Tremors, convulsions, shortness of breath, inhibition of respiratory system.
Safety Profile
Suspected carcinogen with experimental carcinogenic and teratogenic data. Poison by intraperitoneal route. Moderately toxic by ingestion and subcutaneous routes. An experimental teratogen. Mutation data reported. Combustible when exposed to heat or flame. Can react vigorously with oxidizing materials. To fight fre, use alcohol foam, foam, CO2, dry chemical. When heated to decomposition, or on contact with acid or acid fumes, it emits hghly toxic fumes of Cl-. See also CHLOROPHENOLS.
Potential Exposure
2,4-Dichlorophenol is a commercially produced substituted phenol used in the manufacture of industrial and agricultural products; in synthesis of pharmaceuticals. As an intermediate in the chemical industry, 2,4-DCP is utilized as the feedstock for the manufacture of 2,4-dichlorophenoxyacetic acid (2,4-D), and 2,4-D derivatives (germicides, soil sterilants, etc.); certain methyl compounds used in mothproofing, antiseptics and seed disinfectants. 2,4-DCP is also reacted with benzene sulfonyl chloride to produce miticides or further chlorinated to pentachlorophenol, a wood preservative. It is thus a widely used pesticide intermediate. The only group expected to be at risk for high exposure to 2,4-DCP is industrial workers involved in the manufacturing or handling of 2,4-DCP and 2,4-D
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Speed in removing material from skinis of extreme importance. Shampoo hair promptly if contaminated. Seek medical attention immediately. If thischemical has been inhaled, remove from exposure, beginrescue breathing (using universal precautions, includingresuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Carcinogenicity
In mammalian cells in vitro 2,4-DCP produced
chromosomal aberrations and induced
unscheduled DNA synthesis; it was negative
for sister chromatid exchange in vivo and was
mostly negative in bacterial assays.
Oral exposure of pregnant rats to
750mg/kg/day for 10 gestational days induced
slightly decreased fetal weight, delayed ossification
of sternal and vertebral arches, and some
early embryonic deaths.10 Maternal deaths also
occurred at this dose, indicating that 2,4-DCP
was not selectively toxic to embryos or fetuses.
No effects were noted in dams or offspring
exposed at 375mg/kg/day.
A threshold limit value (TLV) has not been
established for 2,4-dichlorophenol.
Environmental Fate
Biological. In activated sludge, 2.8% mineralized to carbon dioxide after 5 d (Freitag et al.,
1985). In freshwater lake sediments, anaerobic reductive dechlorination produced 4-chlorophenol
(Kohring et al., 1989). Chloroperoxidase, a fungal enzyme isolated from Caldariomyces fumago,
converted 9 to 12% of 2,4-dichlorophenol to 2,4,6-trichlorophenol (Wannstedt et al., 1990). When
2,4-dichlorophenol was statically incubated in the dark at 25 °C with yeast extract and settled
domestic wastewater inoculum, significant biodegradation with rapid adaptation was observed. At concentrations of 5 and 10 mg/L, 100 and 99% biodegradation, respectively, were observed after 7
d (Tabak et al., 1981). In activated sludge inoculum, 98.0% COD removal was achieved. The
average rate of biodegradation was 10.5 mg COD/g?h (Pitter, 1976).
Surface Water. Hoigné and Bader (1983) reported 2,4-dichlorophenol reacts with ozone at a rate
constant of <1,500/M?sec at the pH range of 1.5 to 3.0.
Groundwater. Nielsen et al. (1996) studied the degradation of 2,4-dichlorophenol in a shallow,
glaciofluvial, unconfined sandy aquifer in Jutland, Denmark. As part of the in situ microcosm
study, a cylinder that was open at the bottom and screened at the top was installed through a cased
borehole approximately 5 m below grade. Five liters of water was aerated with atmospheric air to
ensure aerobic conditions were maintained. Groundwater was analyzed weekly for approximately
3 months to determine 2,4-dichlorophenol concentrations with time. The experimentally
determined first-order biodegradation rate constant and corresponding half-life were 0.20/d and
3.47 d, respectively.
Photolytic. In distilled water, photolysis occurs at a slower rate than in estuarine waters
containing humic substances. Photolysis products identified in distilled water were the three
isomers of chlorocyclopentadienic acid. The following half-lives were reported for 2,4-
dichlorophenol in estuarine water exposed to sunlight and microbes: 0.6 and 2.0 h during summer
(24 °C) and winter (10 °C), respectively; in distilled water: 0.8 and 3.0 h during summer and
winter, respectively; in poisoned estuarine water: 0.7 and 2.0 h during summer and winter,
respectively (Hwang et al., 1986). When titanium dioxide suspended in an aqueous solution was
irradiated with UV light (λ = 365 nm), 2,4-dichlorophenol was converted to carbon dioxide at a
significant rate (Matthews, 1986). An aqueous solution containing hydrogen peroxide and
irradiated by UV light (λ = 296 nm) converted 2,4-dichlorophenol to chlorohydroquinone and 1,4-
dihydroquinone (Moza et al., 1988). A carbon dioxide yield of 50.4% was achieved when 2,4-
dichlorophenol adsorbed on silica gel was irradiated with UV light (λ >290 nm) for 17 h (Freitag
et al., 1985).
Chemical/Physical. 2,4-Dichlorophenol will not hydrolyze to any reasonable extent (Kollig,
1993). Reported second-order rate constants for the reaction of 2,4-dichlorophenol and singlet
oxygen in water at 292 K: 7 x 106/M?sec at pH 5.5, 2 x 106/M?sec at pH 6, 1.0 x 105/M?sec at pH
6.65, 1.5 x 106/M?sec at pH 7.0, 7.6 x 105/M?sec at pH 7.9, 1.20 x 104/M?sec at pH 9.0 to 9.6. At
pH 8, the half-life of 2,4-dichlorophenol is 62 h (Scully and Hoigné, 1987). In an aqueous
phosphate buffer at 27 °C, 2,4-dichlorophenol reacted with singlet oxygen at a rate of 5.1 x
106/M?sec (Tratnyek and Hoigné, 1991). At neutral pH, 2,4-dichlorophenol was completely
oxidized by potassium permanganate (2.0 mg/L) after 15 min (quoted, Verschueren, 1983).
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with 2,4-DCPyou should be trained on its proper handling and storage.Store in tightly closed containers in a refrigerator awayfrom oxidizers, acid, acid fumes, acid chlorides, acid anhydrides, caustics. A regulated, marked area should be established where this chemical is handled, used, or stored incompliance with OSHA Standard 1910.1045.
Shipping
UN2020 Chlorophenols, solid, Hazard Class: 6.1; Labels: 6.1-Poisonous materials
Purification Methods
Crystallise it from pet ether (b 30-40o). Purify it also by repeated zone melting, using a P2O5 guard tube to exclude moisture. It is very hygroscopic when dry. [Beilstein 6 IV 885.]
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides. Contact with acids or acid fumes causes decomposition releasing poisonous chlorine gas. Incompatible with caustics, acid anhydrides; acid chlorides. Quickly corrodes aluminum; slowly corrodes zinc, tin, brass, bronze, copper and its alloys. May accumulate static electrical charges, and may cause ignition of its vapors.
Waste Disposal
Dissolve in a combustible solvent and incinerate in a furnace equipped with afterburner and scrubber. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≧100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal
Properties of 2,4-Dichlorophenol
| Melting point: | 42-43 °C(lit.) |
| Boiling point: | 209-210 °C(lit.) |
| Density | 1.383 |
| vapor pressure | 1.3 hPa (50 °C) |
| refractive index | 1.4430 (estimate) |
| Flash point: | 237 °F |
| storage temp. | 2-8°C |
| solubility | methanol: soluble1g in 10ml |
| form | melt |
| pka | pK1:7.85 (25°C) |
| color | White to beige |
| Water Solubility | 4.5 g/L (20 ºC) |
| Merck | 14,3072 |
| BRN | 742467 |
| Henry's Law Constant | 2.88 x 10-6 atm?m3/mol at 20 °C (Sheikheldin et al., 2001)
3.23 x 10-6 atm?m3/mol at 25 °C (estimated, Leuenberger et al., 1985a) |
| CAS DataBase Reference | 120-83-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Phenol, 2,4-dichloro-(120-83-2) |
| EPA Substance Registry System | 2,4-Dichlorophenol (120-83-2) |
Safety information for 2,4-Dichlorophenol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H311:Acute toxicity,dermal H314:Skin corrosion/irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2,4-Dichlorophenol
| InChIKey | HFZWRUODUSTPEG-UHFFFAOYSA-N |
2,4-Dichlorophenol manufacturer
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran



You may like
-
 2,4DichloroPhenol 99%View Details
2,4DichloroPhenol 99%View Details -
 2,4-Dichlorophenol 98%View Details
2,4-Dichlorophenol 98%View Details -
 2,4-Dichlorophenol pure CAS 120-83-2View Details
2,4-Dichlorophenol pure CAS 120-83-2View Details
120-83-2 -
 2,4-Dichloro phenol, 98% CAS 120-83-2View Details
2,4-Dichloro phenol, 98% CAS 120-83-2View Details
120-83-2 -
![2,4-Dichlorophenol [for Biochemical Research] CAS 120-83-2](https://img.chemicalbook.in//Content/image/CP5.jpg) 2,4-Dichlorophenol [for Biochemical Research] CAS 120-83-2View Details
2,4-Dichlorophenol [for Biochemical Research] CAS 120-83-2View Details
120-83-2 -
 2-4 Dichlorophenol CASView Details
2-4 Dichlorophenol CASView Details -
 2,4-DICHLOROPHENOL For Synthesis CAS 120-83-2View Details
2,4-DICHLOROPHENOL For Synthesis CAS 120-83-2View Details
120-83-2 -
 2,4-Dichlorophenol CAS 120-83-2View Details
2,4-Dichlorophenol CAS 120-83-2View Details
120-83-2
