2,2'-Anhydro-1-beta-D-arabinofuranosylcytosine hydrochloride
Synonym(s):2,2′-Anhydro-(1-β-D -arabinofuranosyl)cytosine hydrochloride;Cyclo-C;Cyclocytidine
- CAS NO.:10212-25-6
- Empirical Formula: C9H12ClN3O4
- Molecular Weight: 261.66
- MDL number: MFCD00012636
- EINECS: 233-515-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
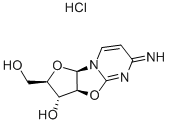
What is 2,2'-Anhydro-1-beta-D-arabinofuranosylcytosine hydrochloride?
Chemical properties
white fine crystalline powder
Originator
Cyclo-C,Kohjin,Japan,1975
The Uses of 2,2'-Anhydro-1-beta-D-arabinofuranosylcytosine hydrochloride
antineoplastic
The Uses of 2,2'-Anhydro-1-beta-D-arabinofuranosylcytosine hydrochloride
Anti-tumor agent
The Uses of 2,2'-Anhydro-1-beta-D-arabinofuranosylcytosine hydrochloride
Ancitabine Hydrochloride is an antineoplastic agent.
What are the applications of Application
Ancitabine hydrochloride is an antineoplastic agent
Definition
ChEBI: A hydrochloride salt resulting from the reaction of equimolar amounts of ancitabine and hydrogen chloride.
Manufacturing Process
A series of reaction steps may be employed in which: (1) Uridine is reacted
with trityl chloride to give 5'-o-trityluridine; (2) Imidazole is reacted with
thiophosgene and that product reacted with the 5'-o-trityluridine to give 2,2'-
anhydro-1-(5'-o-trityl-β-D-arabinofuranosyl)uracil; (3) The preceding uracil
product is converted to the thiouracil using hydrogen sulfide; (4) The trityl
group is removed by treatment with 80% acetic acid; (5) A triacetylated
product is obtained using acetic anhydride; (6) A dithiouracil is prepared from
the uracil intermediate using phosphate pentasulfide.
Preparation of 1-(β-D-arabinofuranosyl)-2-thiocytosine: A solution of 2.0 g of
1-(2',3',5'-O-triacetyl-β-D-arabinofuranosyl)-2,4-dithiouracilin 100 ml of
methanol is saturated with anhydrous ammonia at 0°C. The mixture, in a
glass liner, is heated in a pressure bomb at 100°C for three hours. The
reaction mixture is concentrated to a gum in vacuum, and most of the
byproduct acetamide is removed by sublimation at 60°C/0.1 mm. The residue
is chromatographed on 100 g of silica gel. Elution of the column with
methylene chloride-methanol mixtures with methanol concentrations of 2-25%
gives fractions containing acetamide and a series of brown gums. The desired
product is eluted with 30% methanol-methylene chloride to give a total yield
of 0.386 g (30%), MP 175-180°C (dec.). Recrystallization from methanolisopropanol
furnishes an analytical sample, MP 180-182°C (dec.).
To a solution of 80 mg of 1-(β-D-arabinofuranosyl)-2-thiocytosine in 12 ml of
water is added dropwise 3 ml of a 1 M bromine solution in carbon
tetrachloride. At this point the color of the bromine persists for about 2-3
minutes after each addition. The unreacted bromine is blown off with a stream
of nitrogen, and the reaction mixture is concentrated to a syrup in vacuum
using a bath temperature less than 50°C. The residue is evaporated three
times with 10 ml portions of ethanol, whereupon it crystallizes. The product is
triturated with cold ethanol and with ether to obtain 17 mg of 2,2'-anhydro-1-
(β-D-arabinofuranosyl)cytosine hydrobromide, MP 240°C (dec.).
Treatment of the hydrobromide with a slight excess of ethanolic ammonia
yields the base which may then be converted to the hydrochloride.
Therapeutic Function
Antineoplastic
Properties of 2,2'-Anhydro-1-beta-D-arabinofuranosylcytosine hydrochloride
| Melting point: | 269-270 °C (dec.)(lit.) |
| alpha | -21.8 º (c=2,water) |
| refractive index | -21 ° (C=2, H2O) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | Methanol (Slightly), Water (Sparingly) |
| form | Powder |
| color | White |
| Merck | 14,629 |
Safety information for 2,2'-Anhydro-1-beta-D-arabinofuranosylcytosine hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2,2'-Anhydro-1-beta-D-arabinofuranosylcytosine hydrochloride
| InChIKey | KZOWNALBTMILAP-JBMRGDGGSA-N |
Abamectin manufacturer
Khagga Life Sciences
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 10212-25-6 Ancitabine hydrochloride 98%View Details
10212-25-6 Ancitabine hydrochloride 98%View Details
10212-25-6 -
 Cyclocytidine hydrochloride 95% CAS 10212-25-6View Details
Cyclocytidine hydrochloride 95% CAS 10212-25-6View Details
10212-25-6 -
 Ancitabine Hydrochloride CAS 10212-25-6View Details
Ancitabine Hydrochloride CAS 10212-25-6View Details
10212-25-6 -
 Ancitabine hydrochloride CAS 10212-25-6View Details
Ancitabine hydrochloride CAS 10212-25-6View Details
10212-25-6 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4