Yohimbine hydrochloride
Synonym(s):17α-Hydroxyyohimban-16α-carboxylic acid methyl ester hydrochloride, α₂-Adrenoceptor Antagonist, Yohimbine Hydrochloride;17-Hydroxyyohimban-16-carboxylic acid methyl ester hydrochloride;Yohimbine Hydrochloride - CAS 65-19-0 - Calbiochem
- CAS NO.:65-19-0
- Empirical Formula: C21H27ClN2O3
- Molecular Weight: 390.9
- MDL number: MFCD00012674
- EINECS: 200-600-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
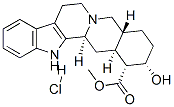
What is Yohimbine hydrochloride?
Chemical properties
white to slightly yellow powder, slightly fragrant, slightly bitter taste, easily soluble in chloroform, soluble in methanol, ethanol, slightly soluble in water.
The Uses of Yohimbine hydrochloride
Yohimbine hydrochloride is an Indole alkaloid with α2-adrenergic blocking activity. It is an alpha adrenergic blocker, mydriatic, antidepressant that used in the treatment of neurological disorders and erectile dysfunction.
The Uses of Yohimbine hydrochloride
Yohimbine hydrochloride is an α2-adrenergic receptor antagonist. It blocks the central α2-adrenegic receptors in the brain, thus preventing and reducing the effects of xylazine, an α2-adrenergic agonist. The sedative effects and respiratory depression that accompany xylazine administration are both antagonized and reversed by yohimbine. Yohimbine hydrochloride also produces an antidiuretic effect, and increases heart rate and blood pressure.
What are the applications of Application
Yohimbine hydrochloride is an α2-adrenoceptor antagonist
General Description
Yohimbine hydrochloride is an indole alkaloid derived from the tree bark. It is an antagonist of α2-adrenergic receptors in the brain, reducing the effects of xylazine, which is an α2-adrenergic agonis.
Biochem/physiol Actions
Yohimbine is derived from the Coryanthe yohimbe tree cortex. It is an alkaloid. Yohimbine hydrochloride is an α2-adrenergic receptor antagonist. It blocks the central α2-adrenergic receptors in the brain, thus preventing and reducing the effects of xylazine, an α2-adrenergic agonist. The sedative effects and respiratory depression that accompany xylazine administration are both antagonized and reversed by yohimbine. Yohimbine hydrochloride also produces an antidiuretic effect, and increases heart rate and blood pressure. It is useful in treating erectile dysfunction and promotes sexual functioning.
Veterinary Drugs and Treatments
Yohimbine is indicated to reverse the effects of xylazine in dogs, but
it is being used clinically in several other species as well.
Yohimbine may be efficacious in reversing some of the toxic effects
associated with other agents (e.g., amitraz) and can be used
prophylactically before amitraz dips.
storage
Store at RT
Properties of Yohimbine hydrochloride
| Melting point: | 288-290 °C (dec.)(lit.) |
| alpha | 99 º (c=1%, H2O, dry subst) |
| refractive index | 103 ° (C=1, H2O) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | H2O: 10 mg/mL |
| form | powder |
| color | white to off-white |
| optical activity | [α]/D 100±5°, c = 1 in H2O |
| Sensitive | Light Sensitive |
| Merck | 14,10102 |
| CAS DataBase Reference | 65-19-0(CAS DataBase Reference) |
| EPA Substance Registry System | Yohimbine hydrochloride (65-19-0) |
Safety information for Yohimbine hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P284:Wear respiratory protection. |
Computed Descriptors for Yohimbine hydrochloride
| InChIKey | PIPZGJSEDRMUAW-VJDCAHTMSA-N |
| SMILES | N1C2=C(C=CC=C2)C2=C1[C@@]1(C[C@]3([C@](CC[C@@H]([C@@H]3C(OC)=O)O)([H])CN1CC2)[H])[H].[H]Cl |&1:9,11,12,15,16,r| |
Yohimbine hydrochloride manufacturer
ENRICH PHARMA
Pharma Links
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran

You may like
-
 Yohimbine hydrochloride 99%View Details
Yohimbine hydrochloride 99%View Details -
 65-19-0 98%View Details
65-19-0 98%View Details
65-19-0 -
 Yohimbine HCl 98% (HPLC) CAS 65-19-0View Details
Yohimbine HCl 98% (HPLC) CAS 65-19-0View Details
65-19-0 -
 Yohimbine hydrochloride CAS 65-19-0View Details
Yohimbine hydrochloride CAS 65-19-0View Details
65-19-0 -
 Yohimbine Hydrochloride CAS 65-19-0View Details
Yohimbine Hydrochloride CAS 65-19-0View Details
65-19-0 -
 Yohimbine hydrochloride CAS 65-19-0View Details
Yohimbine hydrochloride CAS 65-19-0View Details
65-19-0 -
 Yohimbine hydrochloride CAS 65-19-0View Details
Yohimbine hydrochloride CAS 65-19-0View Details
65-19-0 -
 Yohimbine hydrochloride CAS 65-19-0View Details
Yohimbine hydrochloride CAS 65-19-0View Details
65-19-0
