2-Nitrobenzaldehyde
Synonym(s):o-NBA;ortho-nitrobenzaldehyde;2-Nitrobenzaldehyde
- CAS NO.:552-89-6
- Empirical Formula: C7H5NO3
- Molecular Weight: 151.12
- MDL number: MFCD00007132
- EINECS: 209-025-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-20 17:40:16
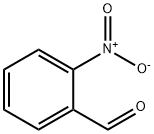
What is 2-Nitrobenzaldehyde?
Chemical properties
yellow or bright yellow needle-like crystals. It can volatilize with water vapor and has the fragrance of benzaldehyde. Soluble in ethanol, ether, benzene, slightly soluble in water, flash point>110℃, reacts violently with pyrrole.
The Uses of 2-Nitrobenzaldehyde
2-Nitrobenzaldehyde is a benzaldhyde with a nitro group substituted in the ortho position. 2-Nitrobenzaldehyde is used in the preparation of dyes and colorants such as Indigo carmine. 2-Nitrobenzaldehyde gas been shown to be a useful photoremovable protecting group as well as in the preparation of more effective ones such as o-Nitrophenylethylene glycol.
What are the applications of Application
It has been shown that 2-Nitrobenzaldehyde (2-NBA) is effective in preventing the generation of single-linear state oxygen in a range of different test samples when exposed to UV and visible light under oxygenated conditions. The oxidation of fatty acids and degradation of photosensitisers were also greatly attenuated by the addition of 2-NBA, suggesting that the presence of 2-NBA has a significant protective effect by limiting the production of mono-linear oxygen species and the photodegradation of dyes. In addition, the compound is highly inhibitory to typical single-linear state oxygen-dependent reactions such as fatty acid photo-oxidation and dye photosensitiser degradation over a rather broad spectral region with wavelengths ranging from 300 nm (UV-B) to 575 nm (near the maximum of ambient solar radiation).
Definition
ChEBI: 2-nitrobenzaldehyde is benzaldehyde substituted at the ortho-position with a nitro group. It is a C-nitro compound and a member of benzaldehydes.
What are the applications of Application
2-Nitrobenzaldehyde can react with chitosan to form 2-nitrobenzyl-chitosan, which is soluble in trifluoroacetic acid and can also be electrospun into nanofiber matrices. On photolysis, the nitrobenzyl group is cleaved to form neat chitosan nanofiber matrices. The same principle has been applied for the preparation of gelatin nanofiber matrices. The condensation of o-NBA with 2-aminobenzothiazole forms o-nitrobenzylidene 2-aminobenzothiazole, a Schiff′s base that can react with metal ions to form complexes.
Preparation
2-Nitrobenzaldehyde is synthesized from 2-Nitrotoluene by nitration and oxidation.
Synthesis of 2-Nitrobenzaldehyde from 2-Nitrotoluene
General Description
The 2-Nitrobenzyl group of 2-nitrobenzaldehyde is photolabile that can be cleaved when exposed to UV-light.
Synthesis
50 g (0.24 mol) of 2-nitrophenylpyruvic acid of melting point 115° C are introduced into 500 ml of aqueous sodium carbonate until a clear solution is obtained. After the addition of 350 ml of toluene, the mixture is cooled to 0° C, and 40 g of solid potassium permanganate is then added in portions at 0°- 3° C. After one hour at 0°- 3° C, 95 ml of 50% strength sulphuric acid are added dropwise. The temperature is not allowed to rise above 30° C. The reaction mixture is filtered, and the toluene phase is separated from the filtrate. The filter residue is washed with toluene, and the combined toluene phases are extracted with 15% strength sodium carbonate solution and with water. The toluene phase is then dried with sodium sulfate and concentrated in a vacuo. The remaining residue consists of 19.7 g (54.7% of theory) of 2-nitrobenzaldehyde, which crystallizes on cooling.
Purification Methods
Crystallise the aldehyde from toluene (2-2.5mL/g) by addition of 7mL pet ether (b 40-60o) for 1mL of solution. It can also be distilled under reduced pressures. [Beilstein 7 IV 584.]
Properties of 2-Nitrobenzaldehyde
| Melting point: | 42-44 °C(lit.) |
| Boiling point: | 153 °C23 mm Hg(lit.) |
| Density | 1,284 g/cm3 |
| vapor density | 5.2 (vs air) |
| refractive index | 1.5800 (estimate) |
| Flash point: | >230 °F |
| storage temp. | Store below +30°C. |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly) |
| form | Crystalline Powder, Needles and/or Chunks |
| color | Yellow |
| Water Solubility | Insoluble in water. |
| Sensitive | Air Sensitive |
| Merck | 14,6586 |
| BRN | 742624 |
| CAS DataBase Reference | 552-89-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzaldehyde, 2-nitro-(552-89-6) |
| EPA Substance Registry System | Benzaldehyde, 2-nitro- (552-89-6) |
Safety information for 2-Nitrobenzaldehyde
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-Nitrobenzaldehyde
2-Nitrobenzaldehyde manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 552-89-6 98%View Details
552-89-6 98%View Details
552-89-6 -
 2-Nitrobenzaldehyde CAS 552-89-6View Details
2-Nitrobenzaldehyde CAS 552-89-6View Details
552-89-6 -
 2-Nitro benzaldehyde CAS 552-89-6View Details
2-Nitro benzaldehyde CAS 552-89-6View Details
552-89-6 -
 2-Nitro benzaldehyde, GR 99%+ CAS 552-89-6View Details
2-Nitro benzaldehyde, GR 99%+ CAS 552-89-6View Details
552-89-6 -
 2-NITROBENZALDEHYDE AR CAS 552-89-6View Details
2-NITROBENZALDEHYDE AR CAS 552-89-6View Details
552-89-6 -
 2-Nitrobenzaldehyde CAS 552-89-6View Details
2-Nitrobenzaldehyde CAS 552-89-6View Details
552-89-6 -
 2-Nitrobenzaldehyde CAS 552-89-6View Details
2-Nitrobenzaldehyde CAS 552-89-6View Details
552-89-6 -
 2-Nitrobenzaldehyde PowderView Details
2-Nitrobenzaldehyde PowderView Details
552-89-6
