2-Nitroacetophenone
- CAS NO.:577-59-3
- Empirical Formula: C8H7NO3
- Molecular Weight: 165.15
- MDL number: MFCD00007144
- EINECS: 209-414-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
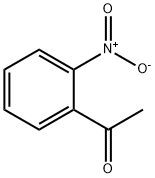
What is 2-Nitroacetophenone?
Chemical properties
yellow-green liquid or crystals. Soluble in alcohol, ether and chloroform, slightly soluble in water.
The Uses of 2-Nitroacetophenone
2'-Nitroacetophenone is an important raw material and intermediate used in Organic Synthesis, Pharmaceuticals, Agrochemicals and dyestuff. It is also used as a synthetic reagent. It is the sensitizer which can be used as pharmaceutical intermediates.
The Uses of 2-Nitroacetophenone
2''-Nitroacetophenone (cas# 577-59-3) is a compound useful in organic synthesis. It is also used as an intermediate for photosensitive materials.?
Preparation
o-Nitroacetophenone is synthesized from o-nitroethylbenzene by oxidation. Add o-nitroethylbenzene, decanoic acid and initiator azobisisobutyronitrile into the reaction tower, and at the same time slowly pass air into the tower, keep the pressure at 0.3-0.8MPa, and control the oxidation at 120-130°C to generate 2-Nitroacetophenone.
Synthesis Reference(s)
The Journal of Organic Chemistry, 43, p. 3631, 1978 DOI: 10.1021/jo00412a058
Journal of the American Chemical Society, 105, p. 943, 1983 DOI: 10.1021/ja00342a050
Synthesis, p. 228, 1988 DOI: 10.1055/s-1988-27522
General Description
Clear yellow liquid. Insoluble in water.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
A nitrated ketone. Ketones are reactive with many acids and bases liberating heat and flammable gases (e.g., H2). The amount of heat may be sufficient to start a fire in the unreacted portion of the ketone. Ketones react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Ketones are incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. They react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4.
Fire Hazard
2-Nitroacetophenone is combustible.
Properties of 2-Nitroacetophenone
| Melting point: | 76-78 °C(lit.) |
| Boiling point: | 202 °C(lit.) |
| Density | 1.23 |
| refractive index | n |
| Flash point: | >230 °F |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Chloroform, Ethyl Acetate |
| form | Solid |
| color | Brown Low Melting |
| Water Solubility | Insoluble |
| FreezingPoint | 24.0 to 28.0 ℃ |
| BRN | 1102322 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 577-59-3(CAS DataBase Reference) |
| NIST Chemistry Reference | Ethanone, 1-(2-nitrophenyl)-(577-59-3) |
| EPA Substance Registry System | 2-Nitroacetophenone (577-59-3) |
Safety information for 2-Nitroacetophenone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for 2-Nitroacetophenone
2-Nitroacetophenone manufacturer
SAKEM LLP
ASM Organics
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 577-59-3 98%View Details
577-59-3 98%View Details
577-59-3 -
 2'-Nitroacetophenone 98%View Details
2'-Nitroacetophenone 98%View Details
577-59-3 -
 577-59-3 3,6-dichloro-4-methylpyridazine 98%+View Details
577-59-3 3,6-dichloro-4-methylpyridazine 98%+View Details
577-59-3 -
 2′-Nitroacetophenone, 95% CAS 577-59-3View Details
2′-Nitroacetophenone, 95% CAS 577-59-3View Details
577-59-3 -
 2'-Nitroacetophenone CAS 577-59-3View Details
2'-Nitroacetophenone CAS 577-59-3View Details
577-59-3 -
 2′-Nitroacetophenone CAS 577-59-3View Details
2′-Nitroacetophenone CAS 577-59-3View Details
577-59-3 -
 2-Nitroacetophenone CAS No. 577-59-3View Details
2-Nitroacetophenone CAS No. 577-59-3View Details
577-59-3 -
 2-Nitroacetophenone 577-59-3 98%View Details
2-Nitroacetophenone 577-59-3 98%View Details
577-59-3
