2-Mercaptopropionic acid
Synonym(s):2-Mercaptopropionic acid;Thiolactic acid
- CAS NO.:79-42-5
- Empirical Formula: C3H6O2S
- Molecular Weight: 106.14
- MDL number: MFCD00004862
- EINECS: 201-206-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
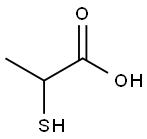
What is 2-Mercaptopropionic acid?
Chemical properties
Clear colorless to slightly yellow liquid. Oily liquid; Melting point 10 ℃; Boiling point d-95 ~ 100 ℃ / 2133Pa, dl- 98.5 ~ 99.0 ℃ / 1866Pa; Density I-D19.24 1.193; Refractive index dl-nD: 1.4823; Optical rotation[α] D-45.47; soluble in water, ethanol and ether.
Chemical properties
Thiolactic acid has a roasted, meaty odor.
The Uses of 2-Mercaptopropionic acid
Depilatory, hair-waving preparations.
The Uses of 2-Mercaptopropionic acid
Thiolactic acid (TLA) can be used as a building block in the synthesis of:
- Thiolactomycin via oxathiolanone intermediate.
- 4-Thiazolidinones by reacting various Schiff bases with thioglycolic acid.
- 1,4-Naphthoquinone derivatives containing sulfur atom for antibacterial and antiviral activity studies.
It can also be used as a bidental chelating agent for the surface modification of titanium dioxide (TiO2) nanoparticles for the removal of cadmium from waste water.
Preparation
By electrolysis of the corresponding sulfide, S(SCHMeCO2H)2.
Definition
ChEBI: 2-mercaptopropanoic acid is a mercaptopropanoic acid.
Taste threshold values
Taste characteristics at 15 ppm: meaty, sulfury, brothy, and brown.
General Description
An oily liquid with an unpleasant odor. Toxic by ingestion and skin absorption. Used as a depilatory and in hair waving preparations.
Air & Water Reactions
Soluble in water and denser than water.
Reactivity Profile
2-Mercaptopropionic acid is an organosulfide/organic acid. Organosulfides are incompatible with acids, diazo and azo compounds, halocarbons, isocyanates, aldehydes, alkali metals, nitrides, hydrides, and other strong reducing agents. Reactions with these materials generate heat and in many cases hydrogen gas. Many of these compounds may liberate hydrogen sulfide upon decomposition or reaction with an acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in 2-Mercaptopropionic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
Safety Profile
Poison by ingestion. Moderately toxic by inhalation. When heated to decomposition it emits toxic fumes of SOx. See also SULFIDES and MERCAPTANS.
Properties of 2-Mercaptopropionic acid
| Melting point: | 10-14 °C (lit.) |
| Boiling point: | 102 °C/16 mmHg (lit.)
203-208 °C (lit.) |
| Density | 1.196 g/mL at 25 °C (lit.) |
| vapor pressure | 1.7 hPa (20 °C) |
| FEMA | 3180 | 2-MERCAPTOPROPIONIC ACID |
| refractive index | n |
| Flash point: | 190 °F |
| storage temp. | 2-8°C |
| solubility | 1g/L in organic solvents at 20 ℃ |
| form | Liquid |
| pka | pK1:4.32(0);pK2:10.20(SH) (25°C) |
| color | Clear colorless to slightly yellow |
| PH | 2 (H2O, 20℃)(undiluted) |
| Odor | at 0.01 % in propylene glycol. meaty sulfurous roasted |
| Water Solubility | MISCIBLE |
| Merck | 14,9340 |
| JECFA Number | 551 |
| BRN | 506218 |
| CAS DataBase Reference | 79-42-5(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Mercaptopropanoic acid(79-42-5) |
| EPA Substance Registry System | Propanoic acid, 2-mercapto- (79-42-5) |
Safety information for 2-Mercaptopropionic acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P363:Wash contaminated clothing before reuse. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for 2-Mercaptopropionic acid
| InChIKey | PMNLUUOXGOOLSP-UHFFFAOYSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2-Mercaptopropionic acid CAS 79-42-5View Details
2-Mercaptopropionic acid CAS 79-42-5View Details
79-42-5 -
 Thiolactic Acid CAS 79-42-5View Details
Thiolactic Acid CAS 79-42-5View Details
79-42-5 -
 2-Mercaptopropionic acid CAS 79-42-5View Details
2-Mercaptopropionic acid CAS 79-42-5View Details
79-42-5 -
 Thiolactic acid CAS 79-42-5View Details
Thiolactic acid CAS 79-42-5View Details
79-42-5 -
 2-Mercaptopropionic acid CAS 79-42-5View Details
2-Mercaptopropionic acid CAS 79-42-5View Details
79-42-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1