2-Mercaptoethanesulfonic acid
- CAS NO.:3375-50-6
- Empirical Formula: C2H6O3S2
- Molecular Weight: 142.2
- MDL number: MFCD00069179
- EINECS: 222-167-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-19 15:17:17
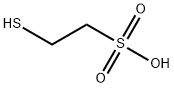
What is 2-Mercaptoethanesulfonic acid?
Absorption
Peak plasma concentrations were reached within 1.5-4 hours for free mesna, and 3-7 hours for total mesna following oral administration. The average oral bioavailability is 58% for free mesna and 89% for total mesna. Food has no effect on the urinary availability of mesna.
Toxicity
The following adverse events were most common (>10%) when mesna was administered with ifosfamide: nausea, vomiting, fatigue, fever, abdominal pain, constipation, diarrhea, leukopenia, anorexia, thrombocytopenia, anemia, granulocytopenia, asthenia, headache, alopecia, and somnolence. Hypersensitivity reactions and dermatologic toxicity may occur in patients taking mesna; therefore, if either reaction occurs, mesna should be discontinued and patient should be provided with supportive care.
Originator
Mistabronco,UCB,W. Germany,1973
Background
Coenzyme M (commonly known by its salt form, Mesna) is a synthetic sulfhydryl (thiol) compound and is used for prophylaxis of Ifosfamide and cyclophosphamide induced hemorrhagic cystitis.
Indications
Mesna is a uroprotective agent and is used prophylactically to reduce ifosfamide and cyclophosphamide induced hemorrhagic cystitis.
Definition
ChEBI: An organosulfonic acid consisting of sulfonic acid having a 2-mercaptoethyl group attached to sulfur.
Manufacturing Process
2,100 g of β-S-thiuronium ethanesulfonate were placed in a solution of 2,100 cc of concentrated aqueous ammonia and 400 cc of water. The mixture was carefully warmed on a steam bath and an exothermic reaction ensured, at which point the β-S-thiuronium ethanesulfonate passed into solution. After standing for two hours at room temperature, the solution was concentrated until all of the excess ammonia had been removed.
The resultant clear solution from the ammonolysis reaction was processed through "Amberlite IR-120" ion exchange resin and converted into β-Smercaptoethanesulfonic acid in 93.7% yield (based on β-S-thiuronium ethanesulfonate).
It is expedient not to heat the reaction mixture rapidly since this increases the loss of ammonia and effects an incomplete reaction. Heating the mixture too rapidly may retard the ammonolysis reaction entirely. The amount of ammonia used is considered to be a satisfactory minimum and larger quantities of ammonia are not found to have any beneficial effect on the reaction. It is also expedient to remove the excess ammonia before processing the guanidinium β-mercaptoethanesulfonate solution through the ion exchange resin since the resin will also remove the ammonia with the result that the capacity of the resin for the exchange of guanidinium ions will be reduced.
Although the preparation of β-mercaptoethanesulfonic acid through the ammonolysis reaction is the preferred method, it is also possible to prepare the sulfonic acid by the sodium hydroxide hydrolysis of β-S-thiuronium ethanesulfonate followed by the ion exchange treatment. The resulting acid,
however, is generally not as satisfactory as that prepared by the ammonolysis
reaction.
Therapeutic Function
Mucolytic
Synthesis Reference(s)
Journal of the American Chemical Society, 77, p. 6231, 1955 DOI: 10.1021/ja01628a052
Pharmacokinetics
Mesna binds to and inactivates acrolein there by preventing or reducing bladder problems
Metabolism
Mesna undergoes rapid oxidation to mesna disulfide (dimesna) which is its major metabolite.
Properties of 2-Mercaptoethanesulfonic acid
| Density | 1.25 g/mL at 20 °C |
| solubility | DMSO |
| form | Powder |
| pka | pK1:;pK2:9.5(-1) (20°C) |
| BRN | 1098878 |
| CAS DataBase Reference | 3375-50-6(CAS DataBase Reference) |
Safety information for 2-Mercaptoethanesulfonic acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P310:Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 2-Mercaptoethanesulfonic acid
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

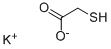

You may like
-
 2-Mercaptoethanesulfonic acid solution CAS 3375-50-6View Details
2-Mercaptoethanesulfonic acid solution CAS 3375-50-6View Details
3375-50-6 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1