2-Mercaptobenzothiazole
Synonym(s):2-Benzothiazolethiol;2-Mercaptobenzothiazole;MBT
- CAS NO.:149-30-4
- Empirical Formula: C7H5NS2
- Molecular Weight: 167.25
- MDL number: MFCD00005781
- EINECS: 205-736-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 14:18:24
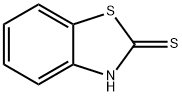
What is 2-Mercaptobenzothiazole?
Description
Mercaptobenzothiazole is a rubber chemical, an accelerant of vulcanization. It is contained in the "mercapto mix". The most frequent occupational categories are metal industry, homemakers, health services and laboratories, building industries, and shoemakers. It is also used as a corrosion inhibitor in cutting fluids or in releasing fluids used in the pottery industry.
Chemical properties
pale yellow monoclinic needle-like or flaky crystals with a disagreeable odor. Insoluble in water and gasoline, soluble in ethanol, ethyl ether, acetone, ethyl acetate, benzene, chloroform and dilute alkali solution.
The Uses of 2-Mercaptobenzothiazole
2-Mercaptobenzothiazole (MBT) is an industrial chemical that is used principally in the manufacture of rubber.Vulcanization accelerator for type of rubber usually used in the production of household rubber gloves rather than medical rubber gloves; corrosion inhibitor in metal-working fluids, detergents, antifreeze, and photographic emulsions. In addition, 2-MBT is formed as a reaction product from some vulcanisation accelerators in elastomer production.
Definition
ChEBI: 2-Mercaptobenzothiazole is a 1,3-Benzothiazole substituted at the 2-position with a sulfanyl group. It is used as a vulcanisation accelerator in the crosslinking of rubber.
What are the applications of Application
2-mercaptobenzothiazole is an accelerator, retarder, and peptizer for natural and other rubber products, but is also used as a corrosion inhibitor in soluble cutting oils and antifreeze mixtures; in greases, adhesives, photographic-film emulsions; detergents; veterinary products, such as tick and flea powders and sprays.It is added to polyether polymers as a stabilizer to resist damage by air and ozone, and is a component approved in the USA in some skin medications for dogs (HSDB, 2015).
2-Mercaptobenzothiazole is also used as an intermediate in the production of pesticides such as 2-(thiocyanomethylthio)benzothiazole (Azam & Suresh, 2012), and sodium and zinc salts of 2-mercaptobenzothiazole are approved for use as pesticides by the EPA (1994).
Preparation
2-Mercaptobenzothiazole is produced by reacting aniline, carbon disulfide, and sulfur at high temperature and pressure; the product is then purified by dissolution in a base to remove the dissolved organics. Re-precipitation is achieved by the addition of acid (Kirk-Othmer, 1982; NTP, 1988).
Refined 2-mercaptobenzothiazole was produced by recrystallization from 2-mercaptobenzothiazole with industrial grade and oxidized to 2,2'-dithiobis(benzothiazole), using oxygen as an oxidant, nitric oxide as a oxygen carrier and alcohols as solvents, in a circulating fluidized reactor under one-step oxidation. 2,2'-Dithiobis(benzothiazole) was thus obtained with high purity up to 99 %, melting point at 183 oC, high yield over 98 %, through the optimization of reaction parameters as reaction time, temperature, reactants ratio, with less waste generation and emission during the production process. Alcohol solvents can be reused after purification.
http://dx.doi.org/10.14233/ajchem.2013.14030
Synthesis Reference(s)
The Journal of Organic Chemistry, 26, p. 3436, 1961 DOI: 10.1021/jo01067a101
General Description
Pale yellow to tan crystalline powder with a disagreeable odor.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
2-Mercaptobenzothiazole is incompatible with strong oxidizing agents. Also incompatible with acids and acid fumes.
Health Hazard
Thiazoles cause allergic skin reactions of type IV [delayed-type hypersensitivity (DTH)].
2-mercaptobenzothiazole is a Standardized Chemical Allergen. The physiologic effect of 2-mercaptobenzothiazole is by means of Increased Histamine Release, and Cell-mediated Immunity.
Fire Hazard
2-Mercaptobenzothiazole is combustible.
Safety Profile
Suspected carcinogen withexperimental carcinogenic and tumorigenic data. Poisonby ingestion and intraperitoneal routes. Experimentalteratogenic and reproductive effects. Mutation datareported. Incompatible with oxidizers. When heated todecomposition
Toxicology
2-Mercaptobenzothiazole has a sensitizing effect, and with chronic exposure (e.g., through the use of rubber gloves) it may induce skin reactions. 2-Mercapto-1-methylimidazole displays teratogenic effects in humans, and it is suspected of being a carcinogen. 6-Mercaptopurine derivatives influence cell division, and have been employed as cytostatic agents. They have been found to be teratogenic in animal studies. Methylthio-substituted triazines, which are used as herbicides, are only slightly toxic. The corresponding LD50 or LC50 values derived from animal studies are above the level of 1000 mg/ kg. Similar values have been established for a methylthiotriazinone that is the active ingredient in the herbicide Sencor.
Carcinogenicity
MBT was not mutagenic in Ames bacterial assays, but it induced chromosomal damage in mammalian cells in culture. Reproductive effects were not observed in two-generation studies of rats treated with up to 15,000 ppm MBT in the diet.
Metabolism
Not Available
Purification Methods
Crystallise it repeatedly from 95% EtOH, or purify it by incomplete precipitation by dilute H2SO4 from a basic solution, followed by several crystallisations from acetone/H2O or *benzene. It complexes with Ag, Au, Bi, Cd, Hg, Ir, Pt, and Tl. [Beilstein 27 II 233, 27 III/IV 2709.]
Properties of 2-Mercaptobenzothiazole
| Melting point: | 177-181 °C(lit.) |
| Boiling point: | 223°C (rough estimate) |
| Density | 1.42 |
| vapor pressure | <0.000003 hPa (25 °C) |
| refractive index | 1.6100 (estimate) |
| Flash point: | 243 °C |
| storage temp. | Store below +30°C. |
| solubility | 0.12g/l |
| form | Powder |
| pka | 9.80±0.20(Predicted) |
| color | Yellow |
| Odor | Odorless |
| PH | 7 (0.12g/l, H2O, 25℃) |
| explosive limit | 15%(V) |
| Water Solubility | <0.1 g/100 mL at 19 ºC |
| Sensitive | Air Sensitive |
| λmax | 325nm(MeOH)(lit.) |
| Merck | 14,5868 |
| BRN | 119484 |
| Stability: | Stable. Incompatible with strong oxidizing agents. Flammable. |
| CAS DataBase Reference | 149-30-4(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Mercaptobenzothiazole(149-30-4) |
| IARC | 2A (Vol. 115) 2018 |
| EPA Substance Registry System | 2-Mercaptobenzothiazole (149-30-4) |
Safety information for 2-Mercaptobenzothiazole
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. |
Computed Descriptors for 2-Mercaptobenzothiazole
| InChIKey | YXIWHUQXZSMYRE-UHFFFAOYSA-N |
2-Mercaptobenzothiazole manufacturer
VPR Rubber
Triveni Interchem Private Limited (Group Of Triveni Chemicals)
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 2-Mercaptobenzothiazole 149-30-4 99%View Details
2-Mercaptobenzothiazole 149-30-4 99%View Details
149-30-4 -
![Benzo[d]thiazole-2(3h)-thione 98% CAS 149-30-4](https://img.chemicalbook.in//Content/image/CP5.jpg) Benzo[d]thiazole-2(3h)-thione 98% CAS 149-30-4View Details
Benzo[d]thiazole-2(3h)-thione 98% CAS 149-30-4View Details
149-30-4 -
 Industrial 2-Mercaptobenzothiazole, 98%View Details
Industrial 2-Mercaptobenzothiazole, 98%View Details
149-30-4 -
 Accelerators Industrial MBT Rubber Chemicals, 149-30-4, Packaging Type: BagView Details
Accelerators Industrial MBT Rubber Chemicals, 149-30-4, Packaging Type: BagView Details
149-30-4 -
 Industrial 2 Mercaptobenzothiazole Mbt, 99.95%View Details
Industrial 2 Mercaptobenzothiazole Mbt, 99.95%View Details
149-30-4 -
 2 - MercaptobenzothiazoleView Details
2 - MercaptobenzothiazoleView Details
149-30-4 -
 2-mercaptobenzothiazole / Mbt / Cas No.149-30-4View Details
2-mercaptobenzothiazole / Mbt / Cas No.149-30-4View Details
149-30-4 -
 2-Mercaptobenzothiazole 149-30-4, BagView Details
2-Mercaptobenzothiazole 149-30-4, BagView Details
149-30-4
