Hexamethylenetetramine
Synonym(s):Hexamethylenetetramine;Hexamine;Methenamine;Urotropine;Hexamethylenetetramine, Hexamine, Formin, Urotropin
- CAS NO.:100-97-0
- Empirical Formula: C6H12N4
- Molecular Weight: 140.19
- MDL number: MFCD00006895
- EINECS: 202-905-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
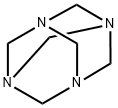
What is Hexamethylenetetramine?
Absorption
After oral administration, rapid absorption of methenamine occurs. (1)
Toxicity
Less than 3.5% of patients treated with this drug will experience minor adverse events such as upset stomach, dysuria, nausea, and rash.
Description
Hexamethylenetetramine is a hardener in epoxy resins of the bisphenol A type and can also be used as an anticorrosive agent. It is a sensitizing agent in ceramics workers.
Chemical properties
White or almost white, crystalline powder or colourless crystals.
The Uses of Hexamethylenetetramine
Hexamethylenetetramine is a versatile reagent in organic synthesis. It is used in the Duff reaction, the Sommelet reaction, and in the Delepine reaction.
The Uses of Hexamethylenetetramine
Used in the treatment of urinary track infection.
The Uses of Hexamethylenetetramine
antibacterial, tuberculostatic
Indications
For prophylactic or suppressive treatment of frequently recurring urinary tract infections when long-term therapy is considered necessary. This drug is not used to treat infection and should only be used after appropriate eradication of infection with antimicrobial agents.
Background
Methenamine is a heterocyclic organic compound with a cage-like structure similar to adamantane. In salt form it is used for the treatment of urinary tract infection (Example: methenamine hippurate which is the hippuric acid salt of methenamine).
What are the applications of Application
Hexamethylenetetramine is a tetraamine compound with structural analogy to adamantane
Definition
ChEBI: A polycyclic cage that is adamantane in which the carbon atoms at positions 1, 3, 5 and 7 are replaced by nitrogen atoms.
Definition
A white crystalline organic compound made by condensing methanal with ammonia. It is used as a fuel for camping stoves, in vulcanizing rubber, and as a urinary disinfectant. Hexamine can be nitrated to make the high explosive cyclonite.
Preparation
Hexamethylenetetramine (also known as hexamine, hexa or HMT) is prepared
from ammonia and formaldehyde:
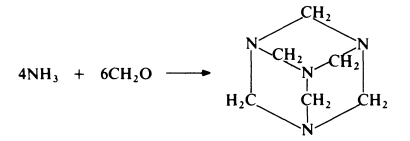
Ammonia is passed into formalin at 20-30??C, with agitation. The resulting solution is evaporated under reduced pressure until most of the water is removed and the hexamethylenetetramine crystallizes. Yields of about 95% on both ammonia and formaldehyde can be achieved. Hexamethylenetetramine is a colourless crystalline solid which, on heating, sublimes with decomposition.
brand name
Uritone (Parke- Davis); Urotropin (Parke-Davis).
General Description
Odorless white crystalline powder or colorless lustrous crystals. Sublimes in a vacuum at about 505° F with some decomposition. Solutions are strong bases (pH of 0.2 molar aqueous solution is 8.4).
Air & Water Reactions
Highly flammable. Burns readily on contact with a flame with a smokeless flame. Finely powdered dust is significant dust explosion hazard. Water soluble.
Reactivity Profile
Hexamethylenetetramine is hygroscopic. Hexamethylenetetramine is sensitive to exposure to heat. Hexamethylenetetramine is incompatible with oxidizing agents. Hexamethylenetetramine is also incompatible with acids. Hexamethylenetetramine reacts violently with sodium peroxide. Hexamethylenetetramine reacts explosively with 1-bromopentaborane(9) at temperatures above 194° F. The complex with iodine deflagrates at 280° F. The 1:1 addition complex with iodoform has exploded at 352° F. Hexamethylenetetramine is corrosive to some metals, such as aluminum and zinc . Special Hazards of Combustion Products: Formaldehyde gas and ammonia may be given off when hot [USCG, 1999].
Hazard
Skin irritant. Flammable, dangerous fire risk.
Fire Hazard
Special Hazards of Combustion Products: Formaldehyde gas and ammonia may be given off when hot.
Flammability and Explosibility
Highly flammable
Pharmaceutical Applications
Methenamine (hexamine, hexamethylenetetraamine), under
the name Urotropin, was successfully used in cystitis by the
German physician Nicolaier in 1895. It has no intrinsic antibacterial
activity and owes its effect to decomposition in acid conditions
to formaldehyde, which is non-specifically microbicidal,
and ammonia. It is often used in the form of organic acid salts,
methenamine hippurate and methenamine mandelate, which
have been claimed (unconvincingly) to keep the urinary pH low.
Mandelic acid has some antibacterial activity in its own right
and is sometimes given alone as a urinary antiseptic, usually
as the calcium or ammonium salt. Infection with urea-splitting
organisms such as Proteus spp. causes the urine to become alkaline
and methenamine is unsuitable for these infections.
Methenamine is absorbed from the gut and mainly excreted
unchanged in the urine, achieving concentrations of around
2–60 mg/L, sufficient to inhibit most bacteria and yeasts.
Higher concentrations are achieved by the hippurate salt.
It is given in enteric-coated tablets to prevent the liberation
of formaldehyde by gastric acid. There is little breakdown in
the blood and no systemic effect or toxicity.
Some patients complain of gastrointestinal upset or frequent
and burning micturition. Attempts to control these side
effects with alkali will abolish the antibacterial effect of the
drug. Contact dermatitis and anterior uveitis have occasionally
been encountered. Prolonged administration or high dosage
may produce proteinuria, hematuria and bladder changes.
Methenamine should not be given to patients with acidosis,
gout or hepatic insufficiency. There have been fears about the
potential carcinogenicity of formaldehyde.
Methenamine and its salts are unsuitable for the treatment
of acute urinary tract infection. Their main use, now largely
supplanted by other agents, has been in the long-term prophylaxis
of recurrent cystitis.
Contact allergens
Hexamethylenetetramine is used in the foundry, tire and rubber, and phenol formaldehyde resins industries and in other applications such as a hardener in epoxy resins Bisphenol A type and as an anticorrosive agent. It is an ammonia and formaldehyde releaser sometimes used in topical medicaments and cosmetics
Pharmacokinetics
Ingestion of a 1-gram dose of methenamine hippurate produces antibacterial activity in the urine within 1/2 hour. Administration of 1 g twice daily produces continuous antibacterial activity in the urine.
Clinical Use
A venerable drug used for the disinfection of acidic urine, methenamine is a low-molecular-weight polymer of ammonia and formaldehyde that reverts to its components under mildly acidic conditions. Formaldehyde is the active antimicrobial component. Methenamine is used for recurrent urinary tract infections. The drug is available in various dosage forms as well as various salts, including the hippurate and mandelate.
Carcinogenicity
No significantly increased incidence
of tumors was observed in rats or mice given HMTA
for their lifetimes. Exposures in rats included 400 mg/day for
1 year, 10,000 ppm in drinking water for 2 years in each
of three generations, 10,000 ppm in water for a lifetime
(261), and up to 1000 ppm in the diet for 2 years.
In mice, testing conditions included up to 10,000 ppm in
drinking water for 60 weeks or 50,000 ppm for 30 weeks and
a lifetime holding period, and up to 10,000 ppm in
the diet for 2 years.
Injection of 25–30 g subcutaneously per mouse led to
an increase in subcutaneous sarcomas in two experiments
(418, 419) but not in two other studies. The
relevance of this methodology to the workplace condition is
questionable.
Metabolism
When in acidic urine (pH<6), methenamine is hydrolyzed to formaldehyde which acts as an antiseptic. (1)
Purification Methods
It is soluble in H2O (67%), CHCl3 (10%), EtOH (8%) and Et2O (0.3%), and a 0.2M solution has a pH of 8.4. Dissolve it in hot absolute EtOH (reflux, Norit), filter using a heated funnel, cool at room temperature first, then in ice. Wash the crystals with cold Et2O, dry them in air or under a vacuum. A further crop can be obtained by adding Et2O to the filtrate. It sublimes above 260o without melting. The picrate has m 179o(dec). [pK2 0 4.85: Reilley & Schmid Anal Chem 30 947 1958, pK2 0 6.30: Pummerer & Hofmann Chem Ber 56 1255 1923.] [Beilstein 26 I 306, 26 II 200, 26 III/IV 1680.]
Properties of Hexamethylenetetramine
| Melting point: | 280 °C (subl.) (lit.) |
| Boiling point: | 246.7°C (rough estimate) |
| Density | 1.33 |
| vapor density | 4.9 (vs air) |
| vapor pressure | <0.01 mm Hg ( 20 °C) |
| refractive index | 1.4260 (estimate) |
| Flash point: | 482 °F |
| storage temp. | Store below +30°C. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Solid |
| pka | 5.1(at 25℃) |
| color | white |
| Odor | Odorless |
| PH | 7-10 (100g/l, H2O, 20℃) |
| Water Solubility | 895 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| Sublimation | 263-295 ºC |
| Merck | 14,5966 |
| BRN | 2018 |
| Stability: | Stable. Incompatible with strong acids, strong oxidizing agents. |
| CAS DataBase Reference | 100-97-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Hexamethylenetetramine(100-97-0) |
| EPA Substance Registry System | Hexamethylenetetramine (100-97-0) |
Safety information for Hexamethylenetetramine
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H228:Flammable solids H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Hexamethylenetetramine
Hexamethylenetetramine manufacturer
Attar Global
Ruban Impex
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran


You may like
-
 Hexamethylenetetramine CAS 100-97-0View Details
Hexamethylenetetramine CAS 100-97-0View Details
100-97-0 -
 Hexamethylenetetramine CAS 100-97-0View Details
Hexamethylenetetramine CAS 100-97-0View Details
100-97-0 -
 Hexamethylenetetramine CAS 100-97-0View Details
Hexamethylenetetramine CAS 100-97-0View Details
100-97-0 -
 Hexamine extrapure AR CAS 100-97-0View Details
Hexamine extrapure AR CAS 100-97-0View Details
100-97-0 -
 Hexamethylenetetramine CAS 100-97-0View Details
Hexamethylenetetramine CAS 100-97-0View Details
100-97-0 -
 Hexamine CASView Details
Hexamine CASView Details -
 Hexamine pure CAS 100-97-0View Details
Hexamine pure CAS 100-97-0View Details
100-97-0 -
 Hexamine 98% CAS 100-97-0View Details
Hexamine 98% CAS 100-97-0View Details
100-97-0
